

Research Article - International Research Journal of Plant Science ( 2023) Volume 14, Issue 2
Received: 09-Jan-2023, Manuscript No. IRJPS-23-86333; Editor assigned: 11-Jan-2023, Pre QC No. IRJPS-23-86333 (PQ); Reviewed: 25-Jan-2023, QC No. IRJPS-23-86333; Revised: 18-Apr-2023, Manuscript No. IRJPS-23-86333 (R); Published: 25-Apr-2023, DOI: 10.14303/IRJPS.2023.050
Pterospermum rubiginosum (P. rubiginosum) are evergreen trees endemic to Southern Western Ghats, popularly used for the treatment of bone fractures as a folklore remedy by tribal population. The physicochemical characterization of the bark powder is the focus of the current investigation. The solvents Methanol, Propanol, Dimethyl Sulfoxide (DMSO), Benzene, and Xylene were used to extract the bark powder and subjected to preliminary phytochemical and antioxidant analysis. Since there have been very few systematic studies on this plant so far, it is vital to standardise the plant based on phytochemical screening of compounds and its antioxidant activity. The findings of qualitative experiments revealed the presence of different classes of phytochemicals and antioxidant properties that are present in the plant bark. The existence of these compounds demonstrates the significance of the plant in the area of novel drug discovery.
Antioxidant activity, Bark extract, Phytochemicals, Pterospermum rubiginosum, Western ghats
People have been seeking out healing plants and herbs to protect themselves from the disease since pre-historic times. The understanding of ethano medicinal plants is the result of numerous human observations and attempts against various diseases, according to a number of works in ancient literature. For instance, it is clear from the Rig Veda that man first began testing various plants on animals before using them on themselves to distinguish these plants as beneficial and toxic.
Following this, medical professionals in tribal and other local communities successively transmitted and practiced these identified medicinally valuable plants and their herbal concoctions as folklore medicine. There are scientific records of tribal communities such as ‘Kani’, a forest dwelling tribe in the Western Ghats. This has also been followed by a majority of households not only in India but throughout the globe, as primary solution/remedy against mild ailments such as fever, cough, cold and other infections and wounds. Since then, they have undergone substantial global investigation and are recognised as important resources for the creation of innovative prospective medications (Petrovska, et al., 2012).
Only a relatively tiny number of plant species out of the overall plant population have so far been examined by ethno botanists and other researchers, despite reports that almost 80% of the world's population relies on traditional medicines as their primary form of healthcare. According to reports, medicinal plants can produce a variety of chemical substances known as secondary metabolites, which have enormous therapeutic value. Majority of these substances have unique anti-inflammatory, antioxidant, or antibacterial properties that can be utilised to treat a variety of disorders. As a result, there are a tonne of flora have had their chemical components examined, which has aided in developing an accurate understanding of their significance and therapeutic potential (Paargavi, et al., 2015). These plant based medications are typically obtained from fruits, seeds, flowers, roots, leaves, bark, latex, and leaves. Alkaloids, tannins, saponins, glycosides, steroids, terpenoids, flavonoids, and lignins are some of the phytochemicals derived from plant and plant products. These phytochemicals include, among others, alkaloids, glycosides, flavonoids, tannins, terpenoids, phenols, steroids, and saponins. By inhibiting the formation of pro-inflammatory signalling molecules, these phytochemicals work as anti-inflammatory medications. Studies have also reported that alkaloids, terpenoids and flavonoids exhibit analgesic properties in plants. The increasing research on medicinal plants become crucial and unavoidable when taking into account the advantageous applications of these phytochemicals, technological advancements as well as interest in switching from conventional pharmaceuticals to natural goods, and other factors (Karunamoorthi, et al., 2013).
Geographical and botanical description of Pterospermum rubiginosum
Taxonomic classification
Kingdom: Plantae
Subkingdom: Tracheobionta
Superdivision: Spermatophyta
Division: Magnoliophyta
Class: Magnoliopsida
Subclass: Dilleniidae
Order: Malvales
Family: Sterculiaceae
Genus: Pterospermum
Species: Rubiginosum
Botanic description
Pterospermum rubiginosum (P. rubiginosum) are evergreen trees that grow upto 20 m high. It has a 5–6 mm thick bark that is flaming red, flaking off in thin rectangular pieces, and brown in colour. The branches have tawny branchlets and are drooping. Simple, alternating leaves have cauducous, lateral, filiform, oblique, thickly pubescent stipules. Petioles are pubescent, 4-6 mm long, and hefty. Lamina is oblong, ovate-lanceolate, or elliptic-ovate, 4-8.5 x 2-3 cm, glabrous above and densely white tomentose beneath, coriaceous; 3-5-ribbed from base, palmate; lateral nerves 4-6 pairs; intercostaes calariform, slender, prominent, pinnate, and noticeable (Mahesh, et al., 2008).
Flowers are white, solitary, bisexual, and have tubular, cylindric, brown-hoary calyxes that split into five linear lobes and are white silky within. The staminal column is attached to the gynophore and bears 5 groups of 3 stamens each between the staminodes, each of which is minutely tubercled toward the tip. There are five white, linear-oblong petals that are somewhat smaller than sepals (Kumar, et al., 2015). Fruits are brown, subclavate, acutely 5-angled, and capsule shaped, with dimensions between 40 and 50 x 6 to 9 mm. The one end of seeds has wings (Sawant, et al., 2014).
Biology
They flower, and the fruits mature from November to April.
Ecology
It is a rare species endemic to Southern Western Ghats, evergreen forests and semi evergreen forests (Chimmini, Kallar, Koruthode, Naduganighats, Kothala, Mukkali forests, Karamanayar region) and is mainly found in districts of Karnataka (Coorg, S. Kanara), Kerala (Idukki, Kollam, Palakkad, Thiruvananthapuram, Wayanad, Thrissur) and Tamil Nadu (Coimbatore, Kanniyakumari, Tiruchchirappalli, Tirunelveli).
Biophysical limits
P.rubiginosum is mostly grown at an altitude of500-700 m.
Vernacular names
The vernacular names are Chittilaplavu, Malamthodali, Ellooti, Sitrilaipolavu, Edinjal and Rusty KanakChampa (Azwanida, et al., 2015).
Traditional uses
Stem bark of the P. rubiginosum is used traditionally for the treatment of bone fracture, injuries, laceration, rheumatism and arthritis by tribal communities such as Kani, Kurichia, Paniya, Kattunaika and Adiyan. Figure 1 Presents examples of the various uses for tree bark (Sindhu, et al., 2012).
Even though it has immense traditional importance in treating several medical conditions, no detailed studies have been reported so far in any literatures related to P. rubiginosum. The bark of P. rubiginosum was examined for its physical and chemical properties as well as the preliminary phytochemical components that would help with future research and a better understanding of the plant's medicinal value (Kagbo, et al., 2010).
Chemicals and reagents
Propanol, Methanol, Xylene, Benzene, Dimethyl sulfoxide (DMSO), Ethanol.
Collection and authenticity
A voucher specimen of Pterospermum rubiginosum bark was deposited in the SNM college international herbarium with the accession number SNMH1002 after being gathered from the Tirunelveli forest area in the Wayanad district of Kerala. Authentication was performed using standard floras for the area (Hassan, et al., 2015).
Plant material preparation
After being cleaned, the bark was shade dried before being put through a grinder to become powder.
Physiochemical characterization of bark sample
The amount of moisture, foreign organic matter, total ash, acid insoluble ash, water soluble ash, crude fiber content, and swelling index were measured in the powdered bark (Altemimi, et al., 2017). The procedures were carried out in accordance with 2011 WHO recommendations for quality control techniques for medicinal plant material (Varghese, et al., 2006).
Moisture content
A petridish containing 5 g of bark powder was weighed, and it was placed in the oven for 18 to 24 hours at 105°C to dry. After being removed from the oven, the samples were let to cool. Once the weight had stabilized, the final measurement was measured (Mathew, et al., 2013). The sample's weight loss was determined as the moisture content using the formulas below:
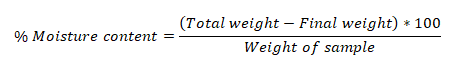
Foreign organic matter
This test was performed by using 100 g of the bark powder (Prasad, et al., 2013). The sample was spread in a thin layer on a clean surface, with visual inspection using naked eye only. The foreign material was separated, weighed and calculated for the percentage content obtained.
Total ash content
A silica crucible that had already been lighted, dried, and tarred was filled with a 4 g sample. The material was then applied evenly to create a thin, slick covering (Vijayan, et al., 2007). Later, it was put over a gas burner with low flame. A temperature of 450°C was adjusted for silica crucibles, and they were allowed to fire until the material became white, signifying the absence of carbon. To calculate the total amount of ash, the crucibles were cooled in desiccators and then weighed. Calculated and presented as percent ash, the overall ash content (Latheef, et al., 2014).
Calculation: Total Ash%=(B-C) x 100/A
Where, A-sample weight in (g) and B-weight of dish+contents after drying (g)
Acid insoluble ash
100 ml of 2% concentrated HCl was prepared. Silica crucible containing the ash was then washed with 2% HCl, the washout collected was again poured into a beaker containing acid and boiled for 10 minutes. It was filtered using Gooch crucible (Borosil) and kept in the oven to dry. The amount of acid insoluble ash was measured in weight (Shyma, et al., 2013).
Calculation: Acid insoluble Ash%=(B-C) x 100/A
Where, A-sample weight in (g), B-weight of dish+contents after drying (g) and C-weight of empty dish (g)
Water soluble ash
Washed silica crucible containing the ash was mixed with water, and the washout was collected. It was dried in the oven after being filtered using a Gooch crucible. The weight of acid insoluble ash was recorded (Nandi, et al., 2016).
Crude fiber content
To remove fat, 5 g of the bark sample was extracted with petroleum ether at temperatures ranging from 35 to 52 degrees Celsius. After being extracted with petroleum ether, 2.5 g of the dry material was heated with 200 ml of sulphuric acid using bumping chips for 30 minutes. It was then rinsed with hot water until the washings were no longer acidic, then filtered through muslin. Later, it was rinsed with 25 ml of boiling 1.25 percent H2SO4, three 50 ml pieces of water, and 25 ml of alcohol before being heated with 200 ml of sodium hydroxide solution for 30 minutes. The leftovers were taken out and placed in an ashtray (pre-weighed dish W1). The leftovers were dried for two hours at 130 ± 2°C, the dish was chilled in a desiccator, and it was then weighed (W2). Then, after cooling in a desiccator and being reweighed, it was ignited for 30 minutes at 600 ± 15°C (W3) (Pradnya, et al., 2014).
Calculation: % crude fiber in sample=Loss in weight on ignition (W2-W1)–(W3-W1) x 100/Weight of the sample
Where, W1 stands for weight of residue before to drying, W2 for weight of residue following 2 hours of drying at 130 ± 2°C, and W3 for weight of residue following 30 minutes of ignition at 600 ± 15°C.
Swelling Index
25 ml of water was put to a measuring cylinder with 1 g of bark powder before being properly mixed.
Then, every 10 minutes to an hour, the mixture was firmly shaken. After that, the combination was left in the cylinder at room temperature for three hours without being touched. The amount of the herbal material, including the sticky mucilage that was contained in the cylinder was measured in millilitres. For 1 g of sample material, the mean value was determined from the individual results (Kodangala, et al., 2010).
Technique for extraction and determination of extractive values
Dried and powdered bark of Pterospermum rubiginosum was extracted utilizing a maceration procedure with Xylene, Benzene, Dimethyl Sulfoxide (DMSO), Propanol, and Methanol. 100 ml of each solvent was added individually to a 250 ml conical flask after 5 g of powdered bark material had been weighed and placed there. Each conical flask has been marked and corked to keep aside for 24 hrs with frequent agitation for first 6 hrs at room temperature. After letting the mixture sit for 18 hours, it is filtered using Whatman No. 1 filter paper. In a shallow dish at 105°C, 25 ml of the filtrate was dried to dryness and weighed (Sasidharan, et al., 2011). The extractive value in percentage was calculated by
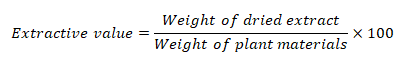
Screening for phytochemicals
Standard protocols were used to conduct preliminary screening of phytochemicals in bark extract samples using Xylene, Benzene, Dimethyl Sulfoxide (DMSO), Propanol, and Methanol. Alkaloids, phytosterols, saponins, carbohydrates, flavonoids, lactones, tannins, proteins, glycosides, phenol, thiol, and phlobatanin substances were all screened for in the extracts (Sheel, et al., 2014).
Froth test: Shaking 5 ml of the extracts with 5 ml of distilled water to create froth, which indicates the presence of saponins, was done.
Detection of phytosterols: Phytosterols are naturally occurring large group of compounds in plants such as trees, herbs or shrubs and are present in variable concentrations in leaves, seeds, branches, roots etc. Food that contains phytosterolsis consumed since they compete with absorption of cholesterol in the digestive system. Therefore, it inhibits the absorption of cholesterol and reduces the blood cholesterol levels (Sparg, et al., 2004).
Solkowski reaction test: A test tube containing 2 ml of bark extract a total of 2 ml of concentrated sulphuric acid and 2 ml of chloroform were introduced from the test tube's sides. The presence of steroids is indicated by the chloroform layer's appearance of a reddish brown colour ring (Matsuura, et al., 2015).
Detection of Carbohydrates-Molisch’s test: Layers were created in the test tube by adding strong sulphuric acid along the sides and mixing 1 ml of bark extract with Molisch reagent. The presence of carbohydrates is shown by the reddish violet ring (Pietta, et al., 2000).
Dragendorff's test: Until an acid reaction takes place, 1 ml of bark extract, 5 ml of distilled water, and 2 M hydrochloric acid were added. Dragendorff's reagent (potassium bismuth iodide) was added in a volume of 1 ml to this. Alkaloids are present when orange or orange-red precipitate forms (Cushnie, et al., 2005).
Detection of lactones-Legal’s test: 1 ml of an extract was placed in a clean test tube, and then sodium nitroprusside, pyridine, and NaOH were added. The presence of lactones is indicated by the rich red colour.
Detection of tannins-Braymer’s Test: 1 ml of an extract, 1 ml of water, and 2-3 drops of ferric chloride were put to a clean test tube. The creation of a green precipitate served as a sign that tannins were present.
Alkaline test: A few drops of diluted NaOH and diluted HCl were added to a clean test tube holding 1 ml of extract. The presence of flavonoids is indicated by the observation of a shift in liquid colour from yellow to colourless.
Detection of phlobatannins: 2 ml of the extract was put to a clean test tube along with 2 ml of the diluted HCl, which was then heated. The presence of phlobatanins is shown by the formation of a red precipitate.
Detection of Thiols: Ammonium sulphate was added to around 0.5 ml of the extract to completely saturate the solution. Then, one or more drops of strong nitric acid were added, followed by 2–4 drops of 5% sodium nitroprusside. Magenta colour production shows the presence of thiol.
Detection of glycosides-Keller-Killiani test: Glacial acetic acid, 5% FeCl3, one drop, and concentrated H2SO4 were all added to 2 ml of plant extract. The existence of glycosides is confirmed by the appearance of a reddish brown colour at the intersection of the two liquid layers and a blue green colour in the top layer.
Detection of phenols-Libermann’s test: A few drops of an aqueous sodium nitrate solution were added after dissolving 1 ml of the extract in 0.5 ml of a 20% Sulphuric acid solution. When diluted, it turned red, and when it was made alkaline with aqueous sodium hydroxide solution, it turned blue.
Detection of saponins: Saponins are compounds widely present in higher plants. They are said to be the main component of majority of folklore medicinal preparations and plant-based drugs as they possess many pharmacological properties. This may include antibacterial, antifungal, antiparasitic, anti-inflammatory, antitumor activity and so on.
Detection of alkaloids: Alkaloids are present in a majority of plant families, thus one among the largest group of compounds identified. Alkaloids from plants serve as defensive substances that shield plants from infections and predators. These substances have anti-inflammatory, antioxidant, and analgesic qualities, among others.
Detection of flavonoids: They are phenolic substances that may be found in a wide range of plants. A variety of functions, including antioxidant, antibacterial, antiviral, anti-inflammatory, and antifungal, are displayed by flavonoids. Ever since the substance was found, it has been researched whether they have any possible antioxidant properties. Humans have employed these flavonoid chemicals for centuries to alleviate ailments and give sustenance. They may be found in vegetables, fruits, seeds, flowers, etc.
Detection of proteins-Biuret test: 10% sodium hydroxide was boiled and applied to a clean test tube containing 1 ml of extract. After heating, 0.7% copper sulphate was additionally added. It was discovered that a purplish violet colour formed, indicating the presence of protein (Anish, et al., 2021).
Determination of total phenolic content
To calculate the total phenolic content of the extracts, the Folin-Ciocalteu reagent technique was utilised. 1 ml of Pterospermum rubiginosum ethanolic bark extract of different concentrations was mixed with 5 ml of Folin-Ciocalteu reagent (1:50 v/v) and 4 ml of 7.5% sodium carbonate. After vigorous shaking for 30 minutes, the mixture's absorbance at 765 nm was measured with a spectrophotometer. The range of polyphenol concentrations calculated from a standard curve of gallic acid is 10 to 100 μg/ml. The total phenolic content of the plant extracts was determined as milligrammes of gallic acid equivalents:
C = (c × V)/m
Where c is gallic acid concentration determined from the calibration curve (mg/ml), V is volume of extract in ml, and m is weight of crude plant extract in g. C=total content of phenolic compounds, mg/g plant extract, in GAE.
Determination of total flavonoid content
The flavonoid content of ethanolic plant bark extract was determined using Aluminium chloride colorimetric method. 1 ml of plant bark extract of various concentrations was mixed with 3 ml methanol, 0.2 ml of 1 M potassium acetate, 0.2 ml of Aluminium chloride and 5.6 ml of double distilled water. The mixture is then shaken vigorously and kept for 30 min at room temperature. The absorbance of the reaction mixture was measured with spectrophotometer at 415 nm. The total flavonoids content of bark extract extracted with ethanol were expressed as milligrams of Quercetin equivalent (QE)/g dry extract (Ranjith, et al., 2018).
C = (c × V)/m
Where C=total content of flavonoid compounds, mg/g plant extract, in quercetin equivalent, c=the concentration of quercetin established from the calibration curve in mg/ml, V=the volume of extract in ml, and m=the weight of crude plant extract in g.
1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging assay
The DPPH radical (1, 1-diphenil-2- picrylhydrazyl) was determined using stock solution of ethanolic bark extract of Pterospermum rubiginosum and L-Ascorbic acid as standard was prepared in the concentration of 1 mg/mL. Varying concentrations from 50-250 μg/mL of bark extract and L-Ascorbic acid was taken respectively and transferred into different tubes and 200 μL by methanol and 0.02 mM of 2 mL DPPH was added and incubated for 20 minutes at dark at room temperature. The absorbance was measured at 517 nm by a spectrophotometer. Free radical destruction activity was measured as a percentage of scavenging:
Percentage of scavenging=(Absorbance of control-absorbance of test)/Absorbance of control*100
Hydroxyl radical scavenging activity
Hydroxyl radical scavenging activity was investigated by using the colorimetric deoxyribose method. The final reaction mixture contains 100 μL of 2-deoxyribose (2.8 mM), KH2PO4.KOH buffer (20 mM pH 7.4), 100 μL FeCl3 (100 μM), 100 μL EDTA, (100 μM), 100 μL of H2O2 (1.0 mM), 100 μL ascorbic acid (100 μM) and concentrations (50 to 250 μg/mL) of the ethanolic extract of Pterospermum rubiginosum bark and L-Ascorbic acid as standard compound. The mixture is then incubated at 37°C for one hour and 0.5 mL of the reaction mixture was taken in a test tube. To produce the colour, 1.5 ml of 2.8% TCA and 1.5 ml of 1% aqueous TBA were added, and the mixture was then incubated at 100°C for 15 minutes. A spectrophotometer was used to detect the absorbance at 530 nm following the incubation. Using quercetin as a positive control. The percentage of scavenging was evaluated by comparing the test and the blank solution (Demiray, et al., 2009).
Percentage of scavenging=(Absorbance of control-absorbance of test)/Absorbance of control*100
Nitric oxide scavenging assay
Utilizing sodium nitroprusside, an assay for nitric oxide scavenging was performed. As a benchmark, L-ascorbic acid is employed. 10 mM 2 ml Sodium Nitroprusside (SNP) in 0.5 ml phosphate buffer saline of pH 7.4 was mixed with 0.5 ml of ethanolic bark extract of Pterospermum rubinosum at various concentrations. The mixture was incubated at 25°C for 3 hours. Following incubation, 0.5 ml was removed and added to 1 ml of the sulphanilamide solution (0.33% in 20% glacial acetic acid), which was then incubated for an additional 5 minutes at room temperature. Following that, 1 ml of 0.1% w/v Griess reagent was mixed and let to sit at room temperature for 30 minutes. The absorbance was measured at 532 nm. Percentage inhibition is calculated according to the formula given below:
Percentage of scavenging=(Absorbance of control-absorbance of test)/Absorbance of control*100
Extraction yield of P. rubiginosum in different solvent extractions
The extractive yield of P. rubiginosum bark from various solvents used to separate the bioactive components of a plant is shown in Table 1. The aqueous extract produced the largest yield (6.48 0.16), while acetone produced the lowest yield (1.75 0.06). High polarity solvent extracts have a high yield percentage, while low polarity solvent extracts have a relatively lower yield %:
| Sl.No. | Solvent | Yield (%w/w) | Color | Consistency |
|---|---|---|---|---|
| 1 | Water | 6.48 ± 0.16 | Brown | Powdery |
| 2 | Ethanol | 4.43 ± 0.09 | Brown | Sticky |
| 3 | Propanol | 3.95 ± 0.05 | Brown | Sticky |
| 4 | DMSO | 2.82 ± 011 | Brown | Sticky |
| 5 | Ethyl acetate | 2.68 ± 0.04 | Brown | Sticky |
| 6 | Acetone | 1.75 ± 0.06 | Brown | Sticky |
| 7 | Chloroform | 2.99 ± 0.08 | Brown | Sticky |
| 8 | Benzene | 3.21 ± 0.08 | Brunette brown | Powdery |
| 9 | Xylene | 2.73 ± 0.07 | Brunette brown | Powdery |
| 10 | nHexane | 2.35 ± 0.11 | Light brown | Sticky |
Table 1. Extractive yield of P.rubiginosum using different solvents.
Physiochemical characterization study
The findings of the thorough physicochemical analysis of the components' moisture content, total ash, crude fiber content, swelling index, acid insoluble ash fractions, etc., were positive and are listed in Table 2.
| Parameters | Results | Unit |
|---|---|---|
| Foreign organic matter | Absent | Percent by weight |
| Total ash content | 10.9 | g/100 g |
| Acid insoluble ash | 0.5 | g/100 g |
| Water soluble ash | 0.9 | g/100 g |
| Crude fibre content | 37.3 | g/100 g |
| Swelling index | 5 | ml/g |
| Moisture content | 2.8 | Percent by weight |
| Total assh content | 10.9 | g/100 g |
Table 2. Physicochemical parameters of P. rubiginosum bark.
Phytochemical screening of P. rubiginosum
The detailed qualitative phytochemical results of plant bark are presented in Table 3. The results of the current research revealed that the extracts contained phenols, saponins, lactones, and flavonoids. Thiols, proteins, tannins and carbohydrates were absent in all the extracts. Non polar solvents contained the highest number of phytochemicals including phytosterols, saponins, lactones, phenols and flavonoids. Phlobatannins and lactones were present in the methanolic extract, whereas saponins and glycosides were present in the propanol fractions (Aryal, et al., 2019).
| SI No. | Phytochemical components | Solvent extracts | ||||
|---|---|---|---|---|---|---|
| Methanol | Propanol | DMSO | Benzene | Xylene | ||
| 1 | Phytosterols | - | - | - | + | + |
| 2 | Saponins | - | + | - | + | + |
| 3 | Carbohydrates | - | - | - | - | - |
| 4 | Flavonoids | + | + | + | + | + |
| 5 | Lactones | + | + | + | + | + |
| 6 | Tannins | - | - | - | - | - |
| 7 | Phenols | + | + | + | + | + |
| 8 | Proteins | - | - | - | - | - |
| 9 | Glycosides | - | + | - | - | - |
| 10 | Thiols | - | - | - | - | - |
| 11 | Phlobatannins | + | - | + | - | - |
Table 3. Qualitative analysis of phytochemical components of Pterospermum rubiginosum (+) denotes the existence of a compound, whereas (-) denotes its absence.
Total phenolic content
Using the Folin-Ciocalteu technique, the total phenolic content of P. rubignosum's ethanolic extract was ascertained. The standard utilised is gallic acid. Table 4 displays the absorbance values at various doses of gallic acid (10 g/mL to 90 g/mL).
The calibration curve was drawn, and the data are presented as gallic acid equivalents (Figure 2). The bark extract's total phenolic content was discovered to be 22.68 g GA/0.5 mg quantity of extract (Blois, et al., 1958).
| SI No. | Concentration of gallic acid (µg/mL) | Mean absorbance (743 nm) |
|---|---|---|
| 1 | 10 | 0.035 ± 0.004 |
| 2 | 30 | 0.200 ± 0.008 |
| 3 | 50 | 0.405 ± 0.004 |
| 4 | 70 | 0.610 ± 0.006 |
| 5 | 90 | 0.901 ± 0.003 |
| Ethanolic extract of bark sample | 0.215 ± 0.013 | |
Table 4. Total Phenolic Content (TPC) of P.rubignosum.
Total Flavonoid Content (TFC)
The regression equation of the calibration curve was used to quantify the total flavonoid content of the ethanolic extract of P. Rubiginosum bark. The measurements are given as micrograms per millilitre of dry sample weight (mg/ml) or as mg Quercetin Equivalents (QE) (Samak, et al., 2009). The total flavonoid content of the plant ethanolic bark extract of P. rubiginosum (EPR) was found that in 500 μl sample contains 11.341 μg QE/0.5 mg mass of extract as shown in Table 5. Most oxidising molecules, including singlet oxygen and a number of other free radicals created during various illnesses, are effectively neutralised by flavonoids (Figure 3) (Mohammad, et al., 2012).
| SI No. | Concentration of quercetin (µg/mL) | Mean absorbance(415 nm) |
|---|---|---|
| 1 | 20 | 0.3561 ± 0.01 |
| 2 | 40 | 0.5564 ± 0.002 |
| 3 | 60 | 0.7201 ± 0.001 |
| 4 | 80 | 0.8890 ± 0.001 |
| 5 | 100 | 1.042 ± 0.003 |
| Ethanolic extract of bark sample | 0.3183 ± 0.003 | |
Table 5. Total Flavanoid Content (TFC) of P.rubignosum.
DPPH free radical scavenging assay
When compared to ascorbic acid, which is utilised as the industry standard, the ethanolic bark extract of P. rubignosum was tested for its capacity to scavenge free radicals. Scavenging activity in the bark extract increased in proportion to concentration, from 50 μg/ml to 250 μg/ml. As shown in Figure 4, the bark extract demonstrated superior free radical scavenging activity when compared to the standard, and the IC50 value was determined to be 163.14 μg/ml. These findings showed that the P. rubiginosum ethanolic extracts are free radical scavengers or inhibitors, perhaps serving as main antioxidants (Phuyal, et al., 2020).
Hydroxyl radical scavenging activity
The most reactive species of all the reduced forms of dioxygen, hydroxyl free radicals are produced in the presence of reduced transition metals like Fe2+and H2O2, and they have the capacity to cause cell damage in living organisms. In the present study inhibition of free radicals of P. rubiginosum bark was evaluated against the standard (Ascorbic acid) and the calibration curve is presented in Figure 5. Dose dependent activity of hydroxyl radical scavenging activity revealed ethanolic bark extract of P. rubiginosum act as antioxidants and are potential hydroxyl radical scavengers. Bark extract's IC50 value is shown to be 151.89 μg/ml. The analysis of the results indicated that the bark extract had larger concentrations of superior antioxidant properties (Bravo, et al., 1998).
Nitric oxide scavenging assay
Nitric oxide is one of the most important chemical mediators which regulate majority of physiological processes. These compounds are generated by macrophages, neurons and endothelial cells. When the level of nitric oxide is too high, cytotoxic consequences have been shown in conditions including cancer, arthritis, and AIDS. The excessive nitric oxide present reacts with oxygen to generate peroxy and nitrite anions that act as free radicals. The results of Nitric oxide method, it proved that are effective as anti-oxidant compared to the ascorbic acid. The IC50 for the ethanolic extract of P. rubiginosum bark was computed, and it was discovered to be more significant than the reference, ascorbic acid. The values were graphically represented in Figure 6 and were observed that as the concentration increases the percentage of scavenging also increases linearly (Agati, et al., 2012).
Phytochemical analysis indicated the presence of various bioactive compounds with diverse chemical groups. Since the bioactivity of a plant is dependent on the presence of secondary metabolites further pharmacological activity studies will be helpful for characterisation of the medicinally important plant bark compounds. The study found that phenols and flavonoids are present indicating that the bark possesses antioxidant potential. The research also demonstrated that the plant's phenol content adds to the bark extract's antioxidant properties. These compounds acts as reducing agents and have the capability of free radical scavenging. Additionally, flavonoids prevent the creation of free radicals by preventing the synthesis of reactive oxygen, chelated trace elements, and reactive species, as well as by enhancing the body's antioxidant defense. Further antioxidant assays like DPPH free radical scavenging assay, hydroxyl radical scavenging activity and nitric oxide scavenging assay confirmed that the extract showed potential antioxidant activity (Subramanian, et al., 2013).
The current study's objective was to evaluate the standards for P. rubiginosum tree bark characterisation. To choose the most efficient solvent for extraction, the value of extraction was evaluated. In the investigation for the characterisation of phytochemicals, phenols, flavonoids, glycosides, and saponins were found. The antioxidant capacity of the bark extract was shown by the presence of phenols and flavonoids. The IC50 value of the ethanolic extract of P. rubiginosum bark was calculated using several antioxidant tests such DPPH, Hydroxyl, and Nitric oxide scavenging techniques. In comparison to the standards, it was discovered that the extract demonstrated considerable antioxidant activity. It also suggested that the bark extract is an excellent therapeutic agent as it can act as primary antioxidants. Further exploratory investigations are required to completely comprehend the chemicals true importance and their mode of action. To identify and describe the active ingredients, pharmacological activity tests against the wound healing property can be performed in-vivo or in-vitro. Antimicrobial activity studies can also be carried out to find its effects on various bacterial and fungal cultures.
I would like to offer my profound gratitude to Dr. Sreejith C.M., the controller of examination at the Mahatma Gandhi university in Kerala, India, for supplying the plant material for the research study and for the support in completing the work.
The authors declare that they have no conflicts of interest.
AKR and TRP designed and performed the phytochemical and antioxidant experiments and KSS and HS compiled the data. AKR processed the data and wrote the manuscript.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Prabha TR, et al. (2023). Phytochemical Analysis and Antioxidant Activity of Bark of Pterospermum rubiginosum. IRJPS. 14: 050.