

Research - African Journal of Food Science and Technology ( 2022) Volume 13, Issue 7
Received: 26-May-2022, Manuscript No. AJFST-22-65078; Editor assigned: 30-May-2022, Pre QC No. AJFST-22-65078; Reviewed: 13-Jun-2022, QC No. AJFST-22-65078; Revised: 08-Jul-2022, Manuscript No. AJFST-22-65078; Published: 15-Jul-2022, DOI: http:/dx.doi.org/10.14303//ajfst.2022.031
Like most powdery foods, cassava-based custard powder (CbCP) and its recipes (yellow-fleshed cassava root starch-YfCRS and whole egg powder-WEP) are hygroscopic, and the storage environmental conditions such as temperature and relative humidity could adversely affect its quality. Thus, this study investigates the moisture adsorption isotherm (MAI) of the CbCP. The CbCP was produced by blending YfCRS (90-98%) with WEP (2-10%) as the major recipes. The CbCP was subjected to MAI using the static gravimetric method at temperatures of 27, 37 and 42 ºC and water activity levels between 0.10 and 0.80. Data obtained were fitted to four sorption models-Peleg, Guggenheim, Anderson, and de Boer (GAB), Oswin and Langmuir. The model fit was evaluated using the coefficient of determination (R2), root means square error (RMSE), and means percentage deviation (%E). The result showed that the Peleg model best fit the CbCP MAI data (0.98 ≤ R2 ≤ 1.00). The monolayer moisture content (Mo) was significantly higher (p < 0.05) in the YfCRS compared to that of the WEP, which was lower. The highest Mo (8.09 g H2O/100g) was obtained in CbCP containing 94% YfCRS and 11.66% WEP, and the lowest monolayer moisture content (6.35 g H2O/100g) was obtained in CbCP containing 98% YfCRS and 10% WEP. However, all the CbCP might be stored for extended periods at all temperatures since their monolayer moisture content falls within the acceptable limit for storage stability.
Cassava-based custard powder, Adsorption isotherm, Monolayer moisture content, Storage stability
Custard powder is a fine-textured unfermented dry food product made from corn starch with other recipes such as salt, flavoring, and coloring agents (Awoyale et al., 2019). As custard powder is a carbohydrate rich breakfast food consumed as a supplement for the sick, there is a need to improve on the micronutrient (vitamin A, iron, and zinc) content to reduce hidden hunger. This might be reduced by replacing corn starch with yellow-fleshed cassava root starch (YfCRS). However, custard powder produced from this YfCRS might be deficient in protein quality and quantity, Hence its supplementation with whole egg powder (WEP). Due to the hygroscopic nature of this product, its interaction with moisture needs to be evaluated for storage stability.
A fundamental property of biological material which is essential for predicting stability and quality changes during packaging and storage of dehydrated foods and formulations, is the moisture adsorption characteristics Moisture adsorption isotherm (MAI) often represents the moisture adsorption characteristic of foods. This isotherm, representing the functional relationship between water activity and equilibrium moisture content of a foodstuff at a given temperature, characterizes the state of water in foods and is of primary interest for several food science and technology applications. The knowledge of moisture sorption isotherm is needed for shelf-life prediction and determination of critical moisture content for acceptability and storage of the product.
From a drying standpoint, adsorption isotherm is required to evaluate the driving force and to define the endpoint of the process, and as well as for modeling and simulation. In addition, the adsorption of water at the surface of a food product may have a physical or chemical origin, and adsorbed water may occupy one or several layers (Mathlouthi & Roge, 2003). There is a dearth of information on the influence of WEP supplementation on the MAI of cassava-based custard powder (CbCP). The interactions of the WEP and YfCRS with water need to be studied in predicting its stability before choosing the suitable packaging material to be used for storage.
Therefore, this work was conducted to evaluate the moisture adsorption isotherm of cassava-based custard powder supplemented with whole egg powder and as a means of predicting the storage stability of the product.
Materials
The yellow-fleshed cassava root (TMS01/1368) was obtained from the cassava farm of the International Institute of Tropical Agriculture (IITA), Ikenne, Ogun State, Nigeria. The TMS01/1368 cassava variety was chosen for this study because of its high β-carotene content (Awoyale et al., 2014). Healthy whole eggs were obtained from Rahmat farm in Gbadeku, Ibadan, Oyo State, Nigeria.
Methods
Production of yellow-fleshed cassava root starch powder
The yellow-fleshed cassava root starch (YfCRS) was produced using the method described by Awoyale et al. (2019). Freshly harvested yellow-fleshed cassava roots were peeled, washed in water, and grated with an electric motor-powered mechanical grater. The resultant pulp was immediately sieved through a muslin cloth and suspended in water. This separates the fibrous and other coarse root material from the starch pulp. The starch pulp was allowed to settle for 4-6 h before decanting. The supernatant containing a mixture of yellow carotenoid and starch was decanted into a bowl, and the thick sediment was the wet starch. The wet starch was reconstituted in water for washing twice, allowed to settle, decanted, and then mixed with the yellow carotenoid, pressed, and dried using a convectional cabinet dryer at 45 ± 5 ºC for 18 h. It was then allowed to cool to room temperature, milled, and packed in polythene nylon prior to further studies.
Production of whole egg powder
The whole egg powder (WEP) was produced using the method described by (Awoyale et al., 2019). Quality whole poultry eggs were carefully washed, dry cleaned, deshelled, and properly homogenized with a metal whisk during which two drops of hydrogen peroxide solution were added to free the products from viable salmonella microorganisms and to prevent browning. The sample was oven-dried at 44 ºC for 4 h and cooled. The egg flakes were scooped, milled, and sieved with a 60 mm mesh and then weighed. The WEP was packaged into a polythene bag for further investigation.
Production of cassava-based custard powder
The cassava-based custard powder (CbCP) was produced using the method described by (Awoyale et al., 2019). Response surface methodology (RSM) of Design-Expert was used to determine the experimental design and the optimal ingredients levels in producing the CbCP. The two basic ingredients used for the custard blends are YfCS (90- 98%) and WEP (2–10%). Other recipes (Sodium chloride and Vanilla flavor) were added. The five coded levels of YfCRS: -α (88.34%), -1 (90%), 0 (94%), +1 (98%), +α (99.66%) and WEP: -α (0.34%), -1 (2 %), 0 (6%), +1 (10%), +α (11.66%) were incorporated into the design and were analyzed in 13 combinations (runs) with two blocks. The central point of the design was repeated five times to calculate the method's reproducibility (Table 1).
| Runs | Yellow-fleshed cassava starch (%) |
Whole egg powder (%) |
|---|---|---|
| 1 | 94.00 | 6.00 |
| 2 | 94.00 | 6.00 |
| 3 | 90.00 | 10.00 |
| 4 | 98.00 | 10.00 |
| 5 | 94.00 | 0.34 |
| 6 | 94.00 | 6.00 |
| 7 | 98.00 | 2.00 |
| 8 | 99.66 | 6.00 |
| 9 | 88.34 | 6.00 |
| 10 | 90.00 | 2.00 |
| 11 | 94.00 | 6.00 |
| 12 | 94.00 | 6.00 |
| 13 | 94.00 | 11.66 |
Table 1. Central composite design of the yellow-fleshed cassava root starch and whole egg powder combinations for cassava-based custard powder formulation.
Determination of moisture adsorption isotherm
The static gravimetric method as reported by (Famurewa et al., 2012), was used to determine the equilibrium moisture content (EMC) of the YfCRS, WEP and CbCP. Triplicate samples of 0.50 ± 0.001 g (MC < 10%) were weighed into moisture pans in the desiccators. The concentrated sulphuric acid quantities used to make up 250 ml of desiccant with deionized water were prepared at 27, 37 and 42°C, using water activity (aw) and temperature tables of Perry and Green (1984). The acid was then dispensed into the desiccators according to their respective aw (Table 2). The desiccators were maintained at aw values of 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7 and 0.8. The desiccators were then placed in a Genlab incubator (Model M75CPD, England) to maintain the required temperature (27, 37 and 42ºC). Each of the samples was weighed every day using a digital balance until a constant weight was obtained in three consecutive recordings, then the sample was assumed to be at equilibrium (± 0.001 g). The time to reach equilibrium ranged between 11 to 23 days depending on the aw in each of the desiccators: those at higher aw reaching equilibrium faster than those at lower aw. The EMC was calculated from which the moisture adsorption isotherms (MAI) were plotted for the samples (Wang N & Brennan JG, 1991).
| Water activities | Quantity of conc. H2SO4/ 250 ml water for 27 °C (ml) |
Quantity of conc. H2SO4/ 250 ml water for 37 °C (ml) |
Quantity of conc. H2SO4/ 250 ml water for 42 °C (ml) |
|---|---|---|---|
| 0.80 | 71.29 | 71.29 | 71.13 |
| 0.70 | 86.28 | 86.79 | 86.92 |
| 0.60 | 99.36 | 100.71 | 101.07 |
| 0.50 | 111.65 | 112.72 | 113.15 |
| 0.40 | 123.30 | 124.55 | 125.11 |
| 0.30 | 135.92 | 137.30 | 137.91 |
| 0.20 | 149.08 | 150.35 | 150.94 |
| 0.10 | 167.61 | 168.99 | 169.63 |
Table 2. Sorption isotherm desiccants preparation for 27, 37 and 42°C.
Isotherm equations and modeling
Four widely recommended isotherm equations [Guggenheim, Anderson and de Boer (GAB), Oswin, Peleg, and Langmuir] were investigated with the experimental data. The mathematical expressions of the models are shown in (Table 3) (Awoyale et al., 2014).
| Models | Equations |
|---|---|
| GAB |  |
| Oswin | 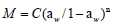 |
| Peleg | 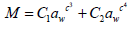 |
| Langmuir |  |
GAB= Guggenheim, Anderson and de Boer equation, M = equilibrium moisture content (% dry basis); a, b, c, n = constant parameters; T = temperature (°C), aw = water activity, Mo= monolayer moisture content.
Table 3. Sorption models used to fit the experimental values.
Statistical analysis
The statistical package for social sciences (SPSS version 15.0 software, SPSS Inc., Chicago, IL) procedure for non-linear regression was used to fit the experimental EMC and aw data in modeling the adsorption isotherms. The accuracy of the models was evaluated using three different indicators, namely Percentage Mean Relative Deviation (%E), Root Mean Square Error (RMSE) and Coefficient of Determination (R2). These indicators of errors are defined as stated in equations (1) and (2).
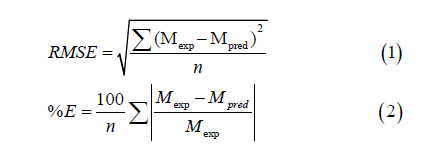
Mexp is the experimental equilibrium moisture content, Mpred is the predicted moisture content and n is the number of observations.
Like most powdery foods, CbCP and its recipe (YfCRS and WEP) are hygroscopic, and the storage environmental conditions such as temperature and relative humidity (RH) could adversely affect its quality (Awoyale et al., 2014). Depicts the effect of temperature on the EMC of YfCRS and WEP. The EMC decreased as the temperature increased for all the samples. The WEP had lower EMC for all the temperatures than the YfCRS, which had higher EMC. The sorption isotherms of the YfCRS and WEP used in the CbCP formulation had a sigmoid shape, typical of the type II isotherm curve going by (Brunauer et al., 1938) classification. The observed decrease in EMC of the YfCRS and WEP at constant aw with increased temperature implied that at any relative humidity (RH), the CbCP could be less hygroscopic with an increase in temperature. Therefore, in an atmosphere of constant RH, the CbCP may be expected to absorb more moisture at lower temperatures than it would at a higher one since the adsorption is an exothermic process (Figure 1).
Subsequently, an increase in temperature at constant moisture content causes a lowering of the isotherm curves. This phenomenon would increase aw, thereby making the product more susceptible to microbial spoilage (Bolin, 1980) (Labuza et al., 1985). In addition, as the temperature increases, sorbed molecules gain kinetic energy and a high degree of freedom. This promotes escape of water from the sorbent surface. This causes the amount of sorbed water to decrease with increased temperature (Labuza et al., 1985). Other researchers reported similar observations for different products (Menkov & Durakova, 2005).
Figures 2-4 show the MAI of the YfCRS and WEP at 42, 37 and 27ºC respectively. The result revealed that the EMC increased with increased water activities (aw) for all samples. The WEP had the lowest EMC for all the aw compared with the YfCRS, which had higher EMC for all the aw. The increased EMC of the YfCRS and WEP with an increase in aw might be because the vapor pressure of water present in the samples increased with that of the surroundings. This observation agreed with the work of (Inchuen et al., 2009) on moisture sorption of Thai curry powder. The observed differences in the EMC of the YfCRS and WEP could be attributed to the variation in the physical adsorption of water on polymeric molecules (Pezzutti & Crapiste, 1997).
It was observed that temperatures, aw, and the blend ratios of YfCRS and WEP had a significant effect (p ≤ 0.001) on the EMC of the CbCP. In addition, the interactions between temperatures and aw (p < 0.01), temperatures and blend ratios (p < 0.001), blend ratios and aw (p ≤ 0.001) as well as that between blend ratios, temperatures and aw (p < 0.001) had significant effects on the EMC of the CbCP respectively The EMC of the blend ratios was high in 94% YfCRS: 11.66% WEP (10.79%) and low in 88.34% YfCRS: 6% WEP (8.62 %). The polymeric molecules present in 94% YfCRS: 11.66% WEP custard powder may tend to absorb more moisture than that of 88.34% YfCRS: 6% WEP because of its high protein content (Aguerre et al., 1983) (Agboluaje, 1989). Hence, custard powder made from 88.34% YfCRS: 6% WEP may have longer shelf life than 94% YfCRS: 11.66% WEP (Johnson, 1998) (Table 4).
| Treatment | Equilibrium moisture content (% dry basis) |
|---|---|
| 94% YfCRS: 0.3%WEP | 9.78 (0.98) d |
| 90% YfCRS: 2% WEP | 9.66 (0.99) e |
| 98%YfCRS: 2% EP | 10.55 (1.03) b |
| 88.34% YfCRS: 6% WEP | 8.62 (0.89) g |
| 94% YfCRS: 6%WEP | 8.97 (0.88) f |
| 99.66% YfCRS: 6% WEP | 10.36 (1.01) c |
| 90 % YfCRS: 10 % WEP | 9.74 (0.85) de |
| 98 % YfCRS: 10% WEP | 8.69 (0.97) g |
| 94% YfCRS: 11.66% WEP | 10.79 (1.01) a |
| Mean | 9.68 |
| p Temp | *** |
| p Water activities | *** |
| p Blend ratios | *** |
| p Temperature x water activities | ** |
| p Temperature x blend ratios | *** |
| p Blend ratios x water activities | *** |
| p Blend ratios x temperature x water activities | *** |
**P<0.01,***P<0.001, ( )=Standard deviation, YfCRS=Yellow-fleshed cassava root starch, WEP=Whole egg powder, EMC=Equilibrium Moisture Content.Means with different superscripts on the same column are significantly different (p < 0.05).
Table 4. Analysis of variance of the effect of temperatures, water activities, and blend ratios of yellow-fleshed cassava root starch and whole egg powder on the equilibrium moisture content of cassava-based custard powder.
The parameters of the various sorption models using the four-sorption equations for CbCP, YfCRS, and WEP are as presented in Table 5. From the results, the Peleg, GAB, and Oswin models had a coefficient of determination (R2) > 90%, and the mean percentage deviation (% E) and root mean square error (RMSE) < 10%, signifying a good fit, while the Langmuir model had R2 < 90%, RMSE< 10% (except for YfCRS, WEP and CbCP of 98% YfCRS: 10% WEP) and % E < 10% (Table 5). However, all the adsorption isotherm models used in this study had a significant variance ratio at p < 0.05 (that is, F-calculated > F-Tabulated) the Peleg model was the most suitable for fitting the moisture sorption data of the CbCP and its recipe (YfCR starch and WEP). This is because the R2 > 90%, and the RMSE and %E are < 10% (Kuye & Ariri, 2005). The order of fitness of the sorption models was Peleg > GAB > Oswin. However, the prediction of the storage stability of the CbCP might be determined using the monolayer moisture content (Mo) (Table 5).
| Samples | Models | A | B | C | D | R2 | RMSE | %E | F-Cal |
|---|---|---|---|---|---|---|---|---|---|
| 94%YfCRS:0.34%WEP | Peleg | 10.31 | 7.20 | 2.73 | 2.38 | 1.00 | 0.00 | 0.00 | 6433.99 |
| GAB | 7.31 | 150.54 | 0.54 | 0.99 | 0.00 | 0.00 | 7916.17 | ||
| Oswin | 9.83 | 0.18 | 0.99 | 0.01 | 0.00 | 12006.00 | |||
| Langmuir | -2.67E+08 | 11.75 | 0.81 | 0.05 | 0.02 | 362.26 | |||
| 90%YfCRS:2%WEP | Peleg | 9.87 | 16.19 | 2.37 | 4.82 | 0.98 | 0.00 | 0.00 | 1984.10 |
| GAB | 6.79 | 1.00E+08 | 0.58 | 0.97 | 0.01 | 0.01 | 1414.87 | ||
| Oswin | 9.64 | 0.19 | 0.96 | 0.01 | 0.01 | 2325.38 | |||
| Langmuir | -1.67E+08 | 12.33 | 0.76 | 0.06 | 0.03 | 269.51 | |||
| 98%YfCRS:2%WEP | Peleg | 8.35 | 11.89 | 5.85 | 0.22 | 0.99 | 0.00 | 0.00 | 888.57 |
| GAB | 8.06 | 210.33 | 0.51 | 0.99 | 0.00 | 0.00 | 4044.36 | ||
| Oswin | 10.56 | 0.18 | 0.98 | 0.01 | 0.00 | 5314.64 | |||
| Langmuir | 8.02 | 14.31 | 0.81 | 0.08 | 0.04 | 495.79 | |||
| 88.34%YfCRS:6%WEP | Peleg | 10.22 | 10.41 | 4.49 | 0.29 | 0.99 | 0.11 | 0.06 | 4544.40 |
| GAB | 6.56 | 95.87 | 0.53 | 0.98 | 0.00 | 0.00 | 2192.55 | ||
| Oswin | 8.69 | 0.19 | 0.98 | 0.01 | 0.00 | 3958.78 | |||
| Langmuir | -6.67E+08 | 10.59 | 0.83 | 0.04 | 0.02 | 383.86 | |||
| 94%YfCRS:6%WEP | Peleg | 9.70 | 4.68E+05 | 1.81 | 19.44 | 0.98 | 0.00 | 0.00 | 4640.61 |
| GAB | 7.09 | 136.52 | 0.45 | 0.97 | 0.01 | 0.00 | 1186.29 | ||
| Oswin | 8.86 | 0.17 | 0.95 | 0.01 | 0.00 | 3622.78 | |||
| Langmuir | 9.47 | 11.64 | 0.82 | 0.06 | 0.03 | 789.24 | |||
| 99.66%YfCRS:6%WEP | Peleg | 11.55 | 8.19 | 0.62 | 4.59 | 0.99 | 0.00 | 0.00 | 27281.48 |
| GAB | 7.69 | 356.87 | 0.54 | 0.99 | 0.00 | 0.00 | 5117.83 | ||
| Oswin | 10.50 | 0.18 | 0.98 | 0.01 | 0.01 | 9374.44 | |||
| Langmuir | -6.67E+08 | 12.46 | 0.81 | 0.05 | 0.02 | 403.74 | |||
| 90%YfCRS:10%WEP | Peleg | 8.98 | 10.56 | 4.81 | 2.23 | 0.99 | 0.00 | 0.00 | 10454.03 |
| GAB | 7.40 | 6.67E+13 | 0.45 | 0.97 | 0.01 | 0.00 | 4427.17 | ||
| Oswin | 9.65 | 0.13 | 0.96 | 0.01 | 0.00 | 6992.79 | |||
| Langmuir | 18.53 | 11.60 | 0.70 | 0.04 | 0.02 | 818.50 | |||
| 98%YfCRS:10%WEP | Peleg | 7.68 | 8.28 | 2.10 | 1.70 | 0.99 | 0.00 | 0.00 | 1624.90 |
| GAB | 6.35 | 191.19 | 0.58 | 0.98 | 0.00 | 0.00 | 4021.56 | ||
| Oswin | 8.90 | 0.20 | 0.98 | 0.01 | 0.00 | 5070.34 | |||
| Langmuir | 6.36 | 12.91 | 0.79 | 0.12 | 0.06 | 335.20 | |||
| 94%YfCRS:11.66%WEP | Peleg | 10.55 | 7.79 | 0.12 | 3.33 | 0.99 | 0.00 | 0.00 | 5408.99 |
| GAB | 8.09 | 266.07 | 0.53 | 0.99 | 0.00 | 0.00 | 6088.10 | ||
| Oswin | 11.02 | 0.17 | 0.98 | 0.01 | 0.00 | 5986.09 | |||
| Langmuir | 8.88 | 14.33 | 0.76 | 0.08 | 0.03 | 412.92 | |||
| TMS01/1368 YfCRS | Peleg | 8.69 | 13.22 | 2.30 | 4.51 | 0.99 | 0.01 | 0.00 | 2549.83 |
| GAB | 6.64 | 71.70 | 0.56 | 0.99 | 0.00 | 0.00 | 2262.16 | ||
| Oswin | 8.81 | 0.22 | 0.98 | 0.01 | 0.00 | 3413.60 | |||
| Langmuir | 4.53 | 13.84 | 0.86 | 0.14 | 0.07 | 472.57 | |||
| WEP | Peleg | 5.64 | 6.16 | 2.94 | 0.23 | 0.98 | 0.00 | 0.00 | 1524.70 |
| GAB | 5.08 | 102.39 | 0.57 | 0.98 | 0.00 | 0.00 | 1845.73 | ||
| Oswin | 6.57 | 0.23 | 0.97 | 0.02 | 0.01 | 2590.73 | |||
| Langmuir | 4.51 | 11.28 | 0.85 | 0.12 | 0.08 | 454.11 |
Peleg (A=C1, B=C2, C=C3,D= C4), GAB (A=Mo, B=b, C=c), Oswin (A=C, n=B), Langmuir (A=C, B=Mo,), YfCRS=Yellow-fleshed cassava root starch, WEP-Whole egg powder, R2=Coefficient of determination, RMSE=Root mean square error, %E=Mean percentage deviation, F=Calculated (0.05) ranged between 5.14 and 6.39.
Table 5. Models for the moisture adsorption isotherm of cassava-based custard powder, yellow-fleshed cassava root starch and whole egg powder at a constant temperature.
Mo is the minimum amount of water bound to active sites and guarantees flour's stability during storage (Iglesias et al., 1975). Table 6 shows the GAB monolayer moisture content (Mo) of the samples. The highest Mo was recorded at 27ºC for YfCRS (7.41 g H2O/100 g solid) and at 37oC for WEP (5.91 g H2O/100 g solid), while the lowest was recorded at 42 ºC for both YfCRS (5.76 g H2O/100 g solid) and the WEP (5.24 g H2O/100 g solid) (Table 6). There was a significant difference (p < 0.05) in the variance ratio of the Mo of the CbCP, YfCRS and WEP at all the storage temperatures as F-calculated was greater than F-tabulated. Mo was significantly higher (p < 0.05) in YfCRS than in the WEP, which was lower. The observed decrease in the Mo of YfCRS and the WEP as the temperature increase could be because the adsorbed molecules gained kinetic energy making the attractive forces to be loosened and this allowed some water molecules to break away from their sorption sites, hence decreasing the Mo (Labuza et al., 1985);(Arevalo-Pinedo et al., 2004). The lower the monolayer values the more the stability of the floury product (Owuamanam et al., 2010). Therefore, the WEP might not provide more binding sites for water molecules and might store for a more extended period as the storage temperature increased up to 42ºC compared to the YfCRS (Owuamanam et al., 2010) (Palou et al., 1997) (Table 6).
| Samples | Temperature (°C) |
Mo (g H2O/100 g solid) |
Mean Mo (g H2O/100 g solid) | F-Cal | F-Tab (0.05) |
|---|---|---|---|---|---|
| 94% YfCRS:0.34% WEP | 42 | 7.01 | 4041.56 | 5.41 | |
| 37 | 7.18 | 9854.27 | 5.41 | ||
| 27 | 7.75 | 7.31 | 9852.68 | 5.41 | |
| 90% YfCRS:2% WEP | 42 | 6.01 | 824.82 | 5.41 | |
| 37 | 6.91 | 2053.90 | 5.41 | ||
| 27 | 7.44 | 6.79 | 1365.90 | 5.41 | |
| 98% YfCRS:2% WEP | 42 | 8.43 | 2626.36 | 5.41 | |
| 37 | 7.59 | 5421.45 | 5.41 | ||
| 27 | 8.16 | 8.06 | 4085.26 | 5.41 | |
| 88.34% YfCRS:6% WEP | 42 | 6.06 | 1106.25 | 5.41 | |
| 37 | 7.10 | 3568.87 | 5.41 | ||
| 27 | 6.51 | 6.56 | 1902.52 | 5.41 | |
| 94% YfCRS:6% WEP | 42 | 7.82 | 1860.33 | 5.41 | |
| 37 | 6.48 | 501.07 | 5.41 | ||
| 27 | 6.97 | 7.09 | 1197.48 | 5.41 | |
| 99.66% YfCRS:6% WEP | 42 | 6.98 | 1901.67 | 5.41 | |
| 37 | 7.56 | 5377.09 | 5.41 | ||
| 27 | 8.55 | 7.70 | 8074.74 | 5.41 | |
| 90% YfCRS:10% WEP | 42 | 7.43 | 8464.26 | 5.41 | |
| 37 | 7.48 | 2189.09 | 5.41 | ||
| 27 | 7.30 | 7.40 | 2628.16 | 5.41 | |
| 98% YfCRS:10% WEP | 42 | 6.32 | 4545.87 | 5.41 | |
| 37 | 6.35 | 1261.68 | 5.41 | ||
| 27 | 6.39 | 6.35 | 6257.12 | 5.41 | |
| 94% YfCRS:11.66% WEP | 42 | 7.84 | 7071.07 | 5.41 | |
| 37 | 8.14 | 1888.66 | 5.41 | ||
| 27 | 8.30 | 8.09 | 9304.58 | 5.41 | |
| TMS01/1368 YfCRS | 42 | 5.76 | 1375.45 | 5.41 | |
| 37 | 6.74 | 2497.43 | 5.41 | ||
| 27 | 7.41 | 6.64 | 2913.60 | 5.41 | |
| WEP | 42 | 5.24 | 3791.17 | 5.41 | |
| 37 | 5.91 | 905.38 | 5.41 | ||
| 27 | 4.09 | 5.08 | 840.62 | 5.41 |
YfCRS=Yellow-fleshed cassava root starch, WEP=Whole egg powder, T=Temperature, Mo= Monolayer moisture content.
Table 6. Guggenheim-Anderson-de Boer (GAB) monolayer moisture content of cassava-based custard powder, yellow-fleshed cassava root starches, and whole egg powder at different temperatures.
The best storage temperature for WEP before use could be 27ºC if properly packaged and stored, and this is because the Mo of WEP at this temperature is low (Owuamanam et al., 2010). Although, the YfCRS and WEP had lower Mo and, thus, might store for an extended period if adequately packaged. The blend ratio of 94% YfCRS: 11.66% WEP (8.09 g H2O/100 g. solid) had the highest Mo while that of 98% YfCRS: 10% WEP (6.35 g H2O/100 g solid) had the least The CbCP with a blend ratio of 98% YfCRS: 10% WEP might store for an extended period if adequately packed in airtight packaging material because of its low Mo. Moreover, the Mo of all the CbCP was within the range for storage stability (< 10% dry basis) as reported by (Labuza et al., 1985). However, there is a need to evaluate the effect of different packaging materials and storage conditions on the MAI of the CbCP (Alakali & Satimehin, 2007).
The adequacy of the model for predicting the CbCP moisture adsorption isotherm is Peleg > GAB > Oswin. The temperatures, aw, and the blend ratios of YfCRS and WEP had a significant effect on the EMC of the CbCP. The highest Mo was recorded at 27ºC for YfCRS and 37ºC for WEP, while the lowest was recorded at 42ºC for YfCRS and the WEP. Mo was significantly higher in YfCRS compared to that of the WEP. The blend ratio of 94% YfCRS: 11.66% WEP (8.09 g H2O/100 g solid) had the highest Mo while 98% YfCRS: 10% WEP (6.35 g H2O/100 g solid) had the lowest. However, the Mo of all the CbCP was within the range for storage stability and may store for an extended period if adequately packed in airtight packaging material. However, there is a need to evaluate the effect of different packaging materials and storage conditions on the MAI of the CbCP.
The cassava breeding unit of the International Institute of Tropical Agriculture (IITA) Ibadan, Nigeria, supplied the yellow-fleshed cassava root used in this study. The incubator at the Genetic Resource Center of IITA, Ibadan, was used for the moisture adsorption isotherm of the samples. I would like to acknowledge the contributions of Profs. L.O.Sanni, T.A. Shittu and A.A Adebowale and Dr. M.O. Adegunwa for their contributions to this work.
The author declared that there is no conflict of interest.
Aguerre RJ, Suarez C, Viollaz PE (1983). Moisture desorption isotherms of rough rice. Int J Food Sci Technol.18: 345-351.
Indexed at, Google Scholar, Cross Ref
Alakali JS & Satimehin AA (2007). Moisture Adsorption Characteristics of Bambara Groundnut (Vigna subterranea) powders. Agric Eng Int CIGR J. 9: 1-15.
Arevalo-Pinedo A, Murr FEX, Giraldo-Zun iga AD, Are valo ZDS (2004). Vacuum drying of carrot (Daucus carota): Effects of pretreatments and parameters process. Drying. 2021-2026.
Awoyale W, Sanni LO, Shittu TA, Adegunwa MO, Gueye B et al., (2014). Varietal effect on the moisture adsorption isotherm of yellow-fleshed cassava root starches. Afr J Root Tuber Crops. 11: 41-49.
Awoyale W, Sanni LO, Shittu TA, Adebowale AA. Adegunwa MO (2019). Development of an optimized cassava starch-based custard powder. J Culin Sci Technol. 17: 22-44.
Indexed at, Google Scholar, Cross Ref
Bolin HR (1980). Retention of the water activity in prumes and raisins. J Food Sci. 45: 1190-1192.
Brunauer S, Emmett P, Teller E (1938). Adsorption of gases in multimolecular layers. J Am Chem Soc 60: 309-319.
Indexed at, Google Scholar, Cross Ref
Famurewa JAV, Oluwamukomi MO, Alaba JO (2012). Storage stability of pupuru flour (a cassava product) at room temperature. Br J Appl Sci Technol. 2: 138-145.
Indexed at, Google Scholar, Cross Ref
Inchuen S, Narkrugsa W, Pornchaloempong P (2009). Moisture sorption of Thai red curry powder. Maejo Int J Sci Technol. 3: 486.
Johnson PNT (1998). Applicability of the BET and GAB models to the moisture adsorption isotherm data of some Ghanaian food flours. Ghana J Agric Sci. 31: 107-112.
Indexed at, Google Scholar, Cross Ref
Kuye A & Ariri I (2005). Modeling the equilibrium moisture sorption data for some Nigerian foods. Int J Food Prop. 8: 1-13.
Indexed at, Google Scholar, Cross Ref
Labuza TP, Kaanene A, Chen JY (1985). Effect of temperature on the moisture sorption isotherms and water activity shift of two dehydrated foods. J Food Sci. 50: 382-391.
Indexed at, Google Scholar, Cross Ref
Mathlouthi M & Roge B (2003). Water vapor sorption isotherms and the caking of food powders. Food Chem. 82: 61-71
Indexed at, Google Scholar, Cross Ref
Menkov MD & Durakova AG (2005). The equilibrium moisture content of Semi-defatted pumpkin seed powder. Int J Food Eng. 1: 1-5.
Indexed at, Google Scholar, Cross Ref
Menkov MD, Durakova AG, Krasteva A (2005). Moisture sorption isotherms of common bean powder at several temperatures. Elec J Env Agricult Food Chem. 4: 892-898.
Owuamanam CI, Iwouno JO, Ihediohanma NC, Barber LI (2010). Cyanide reduction, functional and sensory quality of gari as affected by pH, temperature, and fermentation time. Pak J Nutr. 9: 980-986.
Indexed at, Google Scholar, Cross Ref
Palou E, Lopez–Malo A, Argaiz A (1997). Effect of temperature on the moisture sorption isotherms of some cookies and maize snacks. J Food Eng 3: 85-93.
Indexed at, Google Scholar, Cross Ref
Perry RH & Green D (1984). Chemical Engineers Handbook. sixth edition. McGraw-Hill, New York.
Indexed at, Google Scholar, Cross Ref
Pezzutti A & Crapiste GH (1997). Sorption Equilibrium and Drying Characteristics of Garlic Content. J Food Eng. 31: 113-123.
Indexed at, Google Scholar, Cross Ref
Wang N & Brennan JG (1991). Moisture sorption characteristic of potatoes at four temperatures. J Food Eng. 14: 269-287.
Citation: Awoyale W (2022). Moisture adsorption isotherm of cassava-based custard powder. AJFST. 13: 031.
Copyright: © 2022 International Research Journals This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.