

Full Length Research Article - International Research Journal of Plant Science ( 2024) Volume 15, Issue 1
Published: 29-Feb-2024, DOI: DOI: http:/dx.doi.org/10.14303/irjps.2024.01
Aerva Lanata (L.) Juss, commonly recognized as Mountain Knotgrass in southern India, is renowned for its documented medicinal properties steeped in Hindu mythology, known as Pashanabheda (stone breaker) in Sanskrit and belonging to the Amaranthaceae family, this plant addresses urolithiasis, the formation of stones in the urinary tract. Various plants, including Punarnava (tar vine), Bergenia ligulata, Shigru (Moringa oleifera), Varuna (Crataevanurvala), Kantkari (Solanum Xanthocarpum), Kushmanda seeds (Benincasa hispida), Coriander (Coriandrum sativum), and Jasmine (Jasminum auriculatum), are employed for treating kidney stones. This study focuses on the phytochemical screening of Aerva lanata leaves through extraction using solvents such as Hexane, Ethyl acetate, ethanol, methanol, and water. Employing the Soxhlet extraction method, a conventional technique for extracting phytochemical constituents, the study reveals the presence of tannins, saponins, flavonoids, phenolic compounds, and coumarins. Despite the widespread traditional use of these plants in local areas, the research underscores the medicinal properties found in every part of the Aerva lanata plant, including the stem, leaves, flowers, roots due to that there is by analytical tools such as HPLC, Mass spectroscopy and furthermore carried out antimicrobial studied for each fraction were illustrated. Much needed for researcher to explore phytochemical constituents and identified studied their bioactivities.
Extraction, isolation, analytical instrument and biological studies.
Within diverse natural habitats, numerous medicinal plants play pivotal roles in addressing a wide range of health issues. Yet, a considerable segment of society remains uninformed about these plants and their curative capabilities due to limited awareness. Aerva lanata Juss, popularly referred to as Mount Knotgrass, stands out as one such botanical treasure, teeming with therapeutic attributes. This investigation delves into the isolation and characterization of bioactive compounds from Aerva lanata Juss (Figure 1) and assesses their potential health benefits. Notably, Aerva lanata Juss is esteemed as one of the Dasapushpam(Bhowmik et al., 2012; Goyal et al., 2011; Gr et al., 2013; Haritha et al., 2021; Maharana & Dadhich, 2016; Nirumand et al., 2018; Patel et al., 2011; Sahu et al., 2013), the ten revered flowers of Kerala. Known in Sanskrit as Pashanabedha, which translates to "stone breaker," this plant boasts a rich historical legacy. Traditional uses encompass alleviating headaches, treating skin ailments, facilitating the breakdown of kidney and gallbladder stones, and aiding in uterine cleansing. Through this research, we endeavor to elucidate the therapeutic attributes of Aerva lanata by examining its bioactive compounds and their associated health-promoting effects. (Figure 1)
Systematic position: -
Kingdom: plantae (sub kingdom: tracheobionta)
Clade: tracheophytes, angiosperms, eudicots; Order: Caryophyllales
Family: Amaranthaceae
Class: Magnoliopsida (dicotyledons); Sub class: caryophyllidae
Genus: Aerva; Species: A. lanata;
Division: magnoliophyte (angiosperms, flowering plants).
Known by various names depending on its regional habitat, Aerva Lanata (L.) Juss. has a diverse range of applications in traditional medicine(Musaddiq et al., 2018; Sivasankari et al., 2014). In the realm of Ayurveda, this plant is recognized as a diuretic possessing anti-inflammatory, antibacterial, ant helmintic, and analgesic properties(Pieczykolan et al., 2021) (Rajesh et al., 2011). Furthermore, it is acknowledged for its role in managing diabetes(Gunatilake et al., 2012). Both the leaf and root extracts exhibit anti-inflammatory and antibacterial attributes, commonly utilized in treating skin infections and pustules(Athira & Nair, 2017; Nagaratna et al., 2015). Owing to its esteemed medicinal value, Aerva Lanata has been a focal point of pharmacological and chemical investigations for nearly three decades. Its therapeutic spectrum is attributed to the presence of bioactive compounds like alkaloids, saponins, terpenoids, and various phenolic compounds. Contemporary research has underscored its antioxidant, anti-cancer, anti-inflammatory, and hepatoprotective potentials. The plant's renown extends to its nephroprotective, diuretic, and anti-urolithic properties, which have garnered validation through scientific inquiry. Additionally, recent findings highlight the anti-diabetic attributes of Aerva Lanata root extracts, particularly in enzyme inhibition. However, comprehensive quantitative and qualitative assessments concerning the phenolic acids in Aerva Lanata and their potential correlation with the plant's antioxidant and anti-diabetic activities remain elusive.
Based on the aforementioned insights, our research focused on isolating and identifying the primary bioactive compounds responsible for the plant's diverse range of bioactivities. Additionally, we explored how these bioactive components vary geographically, analyzing their activity levels in different locations. This paper delves into these findings.
Chemical Composition of the plant
Alkaloids
In the examination of relevant literature, it was observed that active compounds such as canthin-6-one alkaloids, including 10-methoxy-canthin-6-one, 10-hydroxy-canthin-6-one, 10-O-β-D-glucopyranosyloxycanthin-6-one, 10-hydroxycanthin-6-one (Ervine), 10-methoxycanthine-6-one (methyl Ervine), 10-β-D-glucopyranosyloxycanthin-6-one (ervoside), aervine (10-hydroxycanthin-6-one), methylaervine (10-methoxycanthin-6-one), and aervoside (10-β-D-glucopyranosyloxycanthin-6-one) were identified in the leaves of Aerva Lanata. Additionally, β-carboline-1-propionic acid, 6-methoxy-β-carboline-1-propionic acid, 6-methoxy-β-carbolin-l-ylpropionic acid (ervolanine), and aervolanine (3-(6-methyoxy- β-carbolin-1-yl) propionic acid) were also found among the alkaloids present in the plant's leaves(Gertruda Zapesochnaya et al., 1992; GG Zapesochnaya, Kurkin, et al., 1991; GG Zapesochnaya, Pervykh, et al., 1991).
Flavonoids
Conversely, Aerva Lanata also serves as a substantial reservoir of flavonoids, including kaempferol, quercetin, isorhamnetin, isorhamnetin 3-O-β-[4-p-coumaroyl-α-rhamnosyl galactoside], and flavanone glucoside persinol, persinosides A and B, 5, 4'-hydroxy-3, 6, 7-trimethoxyflavone, 5-hydroxy-3, 6, 7, 4-tetramethoxyflavone, 5-hydroxy-2',3,5',6,7-pentamethoxyl flavone, 3,3',5,7-trihydroxy-4'-methoxyflavone, apigenin 7-O-β-D-glucoside, and 7-O-β-D-glucopyranoside(Ahmed et al., 2006; Pervykh et al., 1992; Saleh et al., 1990).
Miscellaneous phytoconstituents
Moreover, Aerva Lanata contains additional diverse phytoconstituents, as indicated in various literature sources. These include methyl grevillate, lupeol, lupeol acetate, benzoic acid, β-sitosteryl acetate, and tannic acid. Regarding its nutritive content, the leaves of Aerva Lanata exhibit high levels of carbohydrate (26.6 g/100g), crude protein (22.6 g/100g), and ash (31.2 g/100g). The mineral composition (mg/100g) analysis reveals elevated levels of PO4, along with moderately high concentrations of other minerals such as potassium (K), calcium (Ca), magnesium (Mg), zinc (Zn), and ferrous iron (Fe), while manganese (Mn) content is comparatively lower(Omoyeni & Adeyeye, 2009). (Table 1) (Table 2)
| Bioactive Components | Petrolium Ether |
Benzene | Ethyl Acetate | Methanol | Ethanol |
|---|---|---|---|---|---|
| Alkaloids | + | + | + | + | + |
| Anthroquinones | - | + | - | + | + |
| Catachin | - | + | + | + | + |
| Coumarin | - | + | + | + | + |
| Flovonoids | - | + | + | + | + |
| Phenols | - | - | - | + | + |
| Quinones | + | + | + | + | + |
| Saponins | - | - | - | + | + |
| Steroids | - | + | + | - | + |
| Tannins | - | - | - | + | + |
| Terpenoids | - | + | + | + | + |
| Glycosides | - | - | - | - | - |
| Xanthoprotein | - | + | + | - | + |
| Sugar | + | + | + | + | + |
| Fixed oil | - | - | - | - | - |
| Kaempferol | 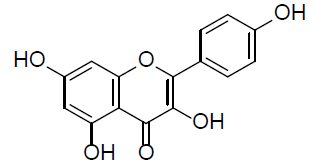 |
| Quercetin | 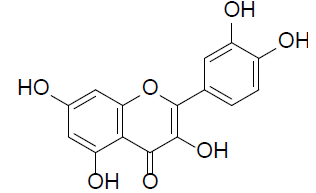 |
| Gallic acid | 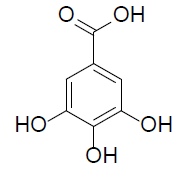 |
| Apigenin | 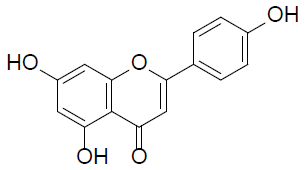 |
| Rutin | 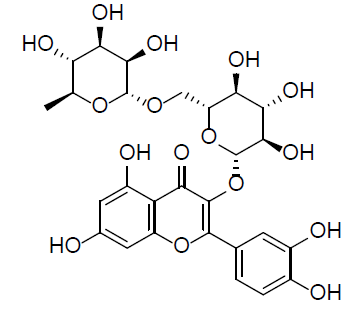 |
| Vanillic Acid | 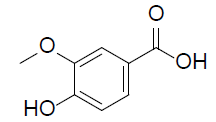 |
| Syringic acid | 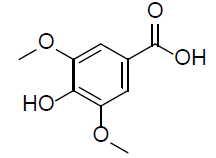 |
| Canthin-6-alkaliods [102] | 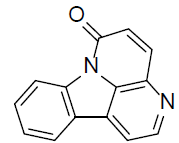 |
| p-coumaric acid | 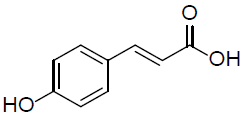 |
| Ferulic acid | 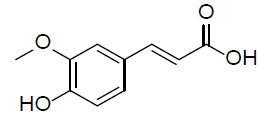 |
| Betanin | 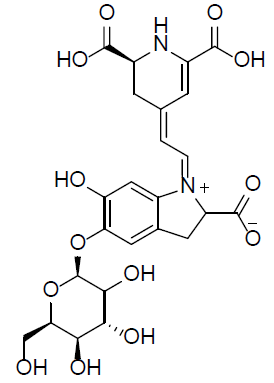 |
Plant Material
In September, leaves of Aerva lanata Juss were harvested from Naganakallu, Kartagi (tq) and preserved in paper bags for about 30 days, shielded from sunlight. Following the drying period, the leaves were ground into a powder using a mortar. The resulting powder was then subjected to extraction using a Soxhlet apparatus to isolate phytochemical components. The solution obtained from the extraction was collected in a conical flask and allowed to undergo solvent evaporation. The resulting residue underwent characterization through various analytical techniques, including HPLC, TLC, Mass spectra analysis, and assessment of Anti-Bacterial activity.
Extract preparation
Pre-extraction selection of plant samples for Soxhlet extraction:
The research centered on the identification of the selected plant, aided by ethnobotanical references from existing literature. Extensive studies in the literature have delved into various plant materials, including leaves, stems, roots, and flowers of Aerva lanata. Our specific emphasis was on the leaves, as we discovered noteworthy macromolecules with significant biomedical applications. Before extraction, adherence to the following criteria was deemed essential.
Selection and collection of plat materials
Efficient isolation of phytoconstituents relies on the careful selection and collection of plant materials. Only disease-free and healthy plants are chosen for extraction, and precautions are taken to shield them from weeds and insects. Various factors come into play during the collection process. The NRCS (Natural Resources Conservation Survey) plant materials program has established guidelines outlining procedures for collecting plant materials, encompassing both seeds and vegetative collections. These guidelines provide details on optimal collection timing, techniques, as well as processing and storage procedures for plant materials.
Drying of plant materials
The extraction of plant materials is significantly influenced by the drying process. Fresh plant materials harbor active enzymes that contribute to the generation of active constituents, intermediates, and metabolic reactions within the plant. Consequently, drying plays a crucial role in the pre-extraction preparation of plant materials. In cases where certain plant materials are sensitive to light and prone to degradation, they are dried in a dark room to mitigate any adverse effects.
Procedure
The Soxhlet extractor setup includes a round-bottom flask, siphon tube, distillation path, condenser, cooling water inlet, cooling water outlet, heat source, and thimble. To initiate the extraction process, 25 grams of plant leaves are precisely weighed and loaded into the thimble, a cotton cloth measuring 15cm in height and 8cm in width. Positioned at the center of the Soxhlet apparatus, the thimble, filled with plant material, is mounted on a heating mantle allowing for temperature adjustment based on solvent boiling points.
As the solvent reaches its boiling point, it vaporizes and travels through the vapor tube, condensing at the condenser. The condensed vapors form droplets of the respective solvents, dripping back into the thimble. Each cycle concludes when the solvent surface surpasses the highest point of the siphon, causing it to drip into the round-bottom flask. Fifteen cycles are completed within 15 minutes, and a total of 60 cycles are observed per hour. This cyclic process is reiterated until the color change in the siphon tube, from yellow to colorless, indicates the endpoint. Subsequently, another solvent, arranged by polarity from non-polar to polar, is introduced through the condenser for subsequent extraction. (Figure 2)
HPLC and Mass Analysis:
We employed a Soxhlet apparatus using a spectrum of solvents ranging from non-polar (hexane) to polar (ethyl acetate, methanol, and water). The resulting crude samples underwent LCMS analysis to discern their components and determine their masses.The identification process revealed a molecular weight of 220 g/mol, validating the presence of canthin-6-alkaloid with a retention time of 1.002 minutes. Additionally, a molecular weight of 286.23 g/mol affirmed the existence of kaempferol at a retention time of 6.713 minutes. Further analysis disclosed a molecular weight of 610.517 g/mol, confirming the presence of rutin at a retention time of 19.497 minutes. In negative mode, a molecular weight of 316.26 g/mol indicated the presence of iso-rhamnetin with a retention time of 13.233 minutes.
Analysis and Quantification of Major Compounds
Graph 1 illustrates the percentage yield of the crude extract obtained through solvent extraction using different solvents in the Soxhlet apparatus. The number of cycles conducted for each solvent includes 940 cycles with hexane, 1080 cycles with ethyl acetate, 11140 cycles with methanol, and 1740 cycles with water. Active phytoconstituents, namely Canthine-6-alkaloid, Rutin, Iso-Rhamnetin, and Kaempferol, were successfully isolated. Graph 2 presents the quantities of these phytoconstituents determined through values obtained from HPLC. Individual isolations of Canthin-6-alkaloid, Rutin, and Kaempferol weighed 3.275 g, 0.697 g, and 0.437 g, respectively. Notably, Canthin-6-alkaloid, Rutin, and Iso-Rhamnetin were isolated from the ethyl acetate extract, while Kaempferol was isolated from the methanol extract. (Figure 3) (Figure 4)
Analysis of Ethyl Acetate Extract
The chromatogram presented here illustrates the HPLC analysis of the ethyl acetate extraction from Aerva Lanata Juss. In this graph, absorbance is plotted against retention time, with an increase in peak height indicating a higher concentration of the compound. The peak area directly reflects the quantity of the compound passing through the detector. Notably, the highest absorption peak is observed at a retention time of 11.58 minutes(S1). For confirmation, molecular weights were employed: a molecular weight of 220 g/mol validated the presence of canthin-6-alkaloid at a retention time of 1.002 minutes(S3), a molecular weight of 610.517 g/mol confirmed the presence of rutin at a retention time of 19.497 minutes(S4), and in negative mode, a molecular weight of 316.26 g/mol affirmed the presence of iso-rhamnetin at a retention time of 13.233 minutes(S5). (Figure 5-9)
Analysis of Methanol Extract
The depicted chromatogram showcases the HPLC analysis of the methanol extraction from Aerva Lanata Juss. In this graph, absorbance is graphed against retention time, where an increased peak height signifies a higher concentration of the compound. The peak area aligns with the detected quantity of the compound. Particularly noteworthy are the highest absorption peaks observed at retention times of 7.95, 8.64, and 10.13 minutes(S6). Confirmation of the presence of kaempferol is solidified with a molecular weight of 286.23 g/mol at a retention time of 6.713 minutes(S8). (Figure 10-12)
The use of UV-Visible spectroscopic method(S9) assists in confirming that the absorption peak at 320 nm is indicative of Kaempferol(Telange et al., 2014), ferulic acid(Pan et al., 2002), and the absorption peak at 410 nm corresponds to Quercetin(Buchweitz et al., 2016).
Anti-Bacterial activity
Experimental Procedure: The antibacterial activity of the plant extraction was directly assessed using the Gram-positive bacterium Staphylococcus aureus. A bacterial culture was cultivated in a peptone broth medium at 28 ± 2°C with continuous agitation at 150 rpm in a rotary shaker. Subsequently, the culture was evenly spread on a Muller-Hinton agar plate, and wells (4 mm diameter) were created using a sterile corkborer. These wells were then filled with 25 µL of the plant extracts, while Streptomycin served as the standard reference. The agar plates were incubated for 12-18 hours at 37°C, following which the zone of inhibition was measured for each extract.Top of Form
Findings: The initial exploration of the antibacterial properties of the plant extraction focused on S. aureus as the model bacterial system. Each plant extract (25 µL) was introduced into a well, and the plate underwent incubation at 37°C. Following approximately 18 hours of incubation, it was observed that the hexane extract exhibited a notably significant zone of inhibition when compared to the other extracts.
This study was undertaken with the objective of isolating bioactive compounds from Aerva Lanata (L.) Juss. Through our investigations, we successfully identified three prominent compounds, namely Kaempferol, ferulic acid, and Quercetin. Utilizing analytical tools such as Mass Spectroscopy, HPLC, and UV-Visible spectrum, we substantiated these identifications with existing literature. Notably, these three compounds were predominantly present in the methanolic and ethanolic extracts.In the context of antibacterial properties, our initial exploration targeted S. aureus as the model bacterial system. The introduction of each plant extract (25 µL) into wells, followed by incubation at 37°C, revealed a significant zone of inhibition with the hexane extract after approximately 18 hours, surpassing the effects observed with other extracts. Ongoing investigations are focused on further exploring the bioactivity of these extracts.
The author is gratified to PG Chemistry, Vijayanagara College Hosapete, for providing well-established research facilities.
The author confirms that there are no known conflicts of interest
Dr. Venkatesh K. Bhovi. Conception, methodology and write up; Mr. K M Karunsagar: isolation, experiments write-up; Dr.Sulochana P Melinmath: write-up, conception
Ahmed, E., Imran, M., Malik, A., & Ashraf, M. (2006). Antioxidant activity with flavonoidal constituents from Aerva persica. Archives of pharmacal research, 29, 343-347.
Indexed at, Google Scholar, Cross Ref
Athira, P., & Nair, S. N. (2017). Pharmacognostic review of medicinal plant Aerva lanata. Journal of pharmaceutical sciences and Research, 9(9), 1420.
Bhowmik, D., Kumar, K. S., Srivastava, S., Paswan, S., & Dutta, A. S. (2012). Traditional Indian herbs Punarnava and its medicinal importance. Journal of pharmacognosy and phytochemistry, 1(1), 52-57.
Buchweitz, M., Kroon, P. A., Rich, G. T., & Wilde, P. J. (2016). Quercetin solubilisation in bile salts: A comparison with sodium dodecyl sulphate. Food chemistry, 211, 356-364.
Indexed at, Google Scholar, Cross Ref
Goyal, M., Pareek, A., Nagori, B. P., & Sasmal, D. (2011). Aerva lanata: A review on phytochemistry and pharmacological aspects. Pharmacognosy reviews, 5(10), 195.
Indexed at, Google Scholar, Cross Ref
Gunatilake, M., Lokuhetty, M. D. S., Bartholameuz, N. A., Edirisuriye, D. T., Kularatne, M. U., & Date, A. (2012). Aerva lanata (Polpala): Its effects on the structure and function of the urinary tract. Pharmacognosy Research, 4(4), 181.
Indexed at, Google Scholar, Cross Ref
Haritha, C., Ramya, D., Naveen, R., Prasanna, S. V., & Salomi, P. (2021). A Comprehensive Review on Bergenia ligulata (Paashanbheda) and its role in the treatment of kidney stone formation. Int. J. Res. Ayurveda Pharm, 12, 94-99.
Indexed at, Google Scholar, Cross Ref
Maharana, L., & Dadhich, O. P. (2016). Review on role of herbal drug in the prevention and management of kidney disease. Ayushdhara Journal, 3(1), 500-508.
Musaddiq, S., Mustafa, K., Ahmad, S., Aslam, S., Ali, B., Khakwani, S., ... & Jabbar, A. (2018). Pharmaceutical, ethnopharmacological, phytochemical and synthetic importance of genus Aerva: A review. Natural Product Communications, 13(3), 1934578X1801300326..
Indexed at, Google Scholar, Cross Ref
Nagaratna, A., Hegde, P. L., & Harini, A. (2015). A Pharmacological review on Gorakha ganja (Aerva lanata (Linn) Juss. Ex. Schult). Journal of Pharmacognosy and Phytochemistry, 3(5), 35-39.
Nirumand, M. C., Hajialyani, M., Rahimi, R., Farzaei, M. H., Zingue, S., Nabavi, S. M., & Bishayee, A. (2018). Dietary plants for the prevention and management of kidney stones: preclinical and clinical evidence and molecular mechanisms. International journal of molecular sciences, 19(3), 765.
Indexed at, Google Scholar, Cross Ref
Omoyeni, O. A., & Adeyeye, E. I. (2009). Chemical composition, calcium, zinc and phytate interrelationships in Aerva lanata (Linn) Juss. ex Schult leaves. Oriental Journal of Chemistry, 25(3), 485..
Patel, R. K., Patel, S. B., & Shah, J. G. (2011). Anti-urolithiatic activity of ethanolic extract of seeds of Benincasa hispida (Thumb). Pharmacologyonline, 3(2011), 586-591.
Pieczykolan, A., Pietrzak, W., Gawlik-Dziki, U., & Nowak, R. (2021). Antioxidant, Anti-Inflammatory, and Anti-Diabetic Activity of Phenolic Acids Fractions Obtained from Aerva lanata (L.) Juss. Molecules, 26(12), 3486.
Indexed at, Google Scholar, Cross Ref
Rajesh, R., Chitra, K., & Paarakh, P. M. (2011). Aerva lanata (Linn.) Juss. ex Schult.–An overview.
Sahu, A. R., Rath, S. C., & Panigrahi, J. (2013). In vitro propagation of Aerva lanata (L.) Juss. ex Schult. through organogenesis.
Saleh, N. A., Mansour, R. M., & Markham, K. R. (1990). An acylated isorhamnetin glycoside from Aerva javanica. Phytochemistry, 29(4), 1344-1345.
Indexed at, Google Scholar, Cross Ref
Sivasankari, B., Anandharaj, M., & Gunasekaran, P. (2014). An ethnobotanical study of indigenous knowledge on medicinal plants used by the village peoples of Thoppampatti, Dindigul district, Tamilnadu, India. Journal of ethnopharmacology, 153(2), 408-423.
Indexed at, Google Scholar, Cross Ref
Telange, D. R., Patil, A. T., Tatode, A., & Bhoyar, B. (2014). Development and Validation of UV Spectrophotometric Method for the Estimation of Kaempferol in Kaempferol: Hydrogenated Soy PhosphatidylCholine (HSPC) Complex. Pharmaceutical Methods, 5(1).
Indexed at, Google Scholar, Cross Ref
Zapesochnaya, G., Kurkin, V., Okhanov, V., & Miroshnikov, A. (1992). Canthin-6-one and β-carboline alkaloids from Aerva lanata. Planta medica, 58(02), 192-196.
Indexed at, Google Scholar, Cross Ref
Zapesochnaya, G. G., Kurkin, V. A., Okhanov, V. V., Perzykh, L. N., & Miroshnilov, A. I. (1991). Structure of the alkaloids of Aerva lanata. Chemistry of Natural Compounds, 27, 725-728.
Indexed at, Google Scholar, Cross Ref
Zapesochnaya, G. G., Pervykh, L. N., & Kurkin, V. A. (1991). A study of the herb Aerva lanata. III. Alkaloids. Chemistry of Natural compounds, 27, 336-340.