

Research Article - International Research Journal of Plant Science ( 2021) Volume 12, Issue 4
, DOI: http:/dx.doi.org/10.14303/irjps.2021.21
Proteins form the class of biological macromolecules that have the most well defined physico-chemical properties and consequently they are easier to isolate and characterize than nucleic acids. Gel electrophoresis is a useful tool for characterizations of plant proteins. The recognitions of protein using electrophoresis is necessary and valuable for taxonomic studies in plants, animals, microorganisms and the viroids. Differential banding pattern for protein in Citrullus Colocynthis (Linn.) Schrad. in all in vivo (leaf, stem, fruit and root) and in vitro (callus and differentiating callus) samples was observed. In vivo samples (leaf, stem, fruit and root) showed three bands while in vitro (callus and differentiating callus) samples showed five bands. Variation in intensity of protein bands was also observed in all samples. Fruit and in vitro samples showed more intense banding pattern. Rm value of first upper band showed marginal variation in all samples (leaf, stem, fruit, root, callus and differentiating callus).
Protein, Gel Electrophoresis, Citrtullus Colocynthis, in vivo, in vitro, Rm value.
Proteins are macromolecular because of their very high molecular weight. These are the polymers, i.e. chain like molecules produced by joining a number of small units of amino acids called monomers. The amino acids are therefore, regarded as “building blocks of proteins”.
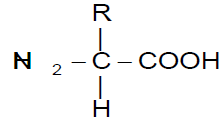
R is the functional group of the amino acid
Each amino acid is a nitrogenous compound having both an acidic carboxyl (-COOH) and a basic amino (-NH2) group. R can be as simple as a hydrogen atom (H) or a methyl group (-CH3) or a more complex structure. Among 1.5 million species of living organisms, there are probably 1010 to 1012 different kinds of protein molecules and about 1010 different kinds of nucleic acids.
Proteins are most abundant intracellular macromolecules and constitute over half the dry weight of most organisms. Proteins occupy a central position in the architecture and functioning of living matter. They are intimately connected with all phases of chemical and physical activity, that constitute the life of the cell.
Gel electrophoresis is a useful tool for characterizations of plant proteins. The recognitions of protein using electrophoresis is necessary and valuable for taxonomic studies in plants, animals, microorganisms and the viroids (Van den Berg, 2002, Arrel & Kalman, 2016).
Almost all analytical electrophoresis of proteins are carried out in polyacrylamide gels under conditions that ensure dissociation of proteins into their individual polypeptides subunits and that minimize aggregation. Polyacrylamide gels are formed by polymerizing acrylamide with a cross linking agent in the presence of a catalyst (persulphate ion) and a chain initiator, TEMED (N´, N´, N´, N´-Tetramethyl ethylene diamine).
SDS-polyacrylamide gel electrophoresis is carried out with a discontinuous buffer system in which the buffer in the reservoirs is of a pH and ionic strength different from that of the buffer is used to cast the gel. SDS is an anionic detergent, which binds strongly to denature proteins. At pH 7.0 in the presence of 1% w/v SDS and 0.2 ml mercaptoethanol, proteins dissociate into their sub units and bind to large quantities of the detergent. The number of SDS molecules bound to a polypeptide chain is approximately half the number of amino acid residues in that chain. When electrophoresis is performed in a gel, the mobility depends on the size of the molecule and on its net charge. Small highly charged molecules move faster than large, less charged ones. Generally, the mobility of the proteins in the presence of SDS is a linear function of the logarithm of the apparent molecular weight.
SDS PAGE recovers hydrophobic proteins and is an important pre requisite for proteome analysis but is not compatible with analysis of proteins in native state (Heinemeyer et al., 2007). According to Chevalier (2007) staining of gel constitutes a crucial step in comparative protein analysis. Gel electrophoresis can directly equate variations in protein banding pattern to genes coding various proteins (Gottlieb, 1971).
Gel electrophoresis has shown that many isozymes and polymorphic proteins are widely distributed in plants (Cherry & Ory, 1972) and the protein polymorphism signals the existence of allelism (Goodenough, 1978). This technique has been widely used to study the protein diversity in many plant species i.e. Lathyrus sativus (Alba et al., 2001); Brachiaria species (Neto et al., 2002); Pasteurella multocida (Tomer et al., 2002); Solanum tuberosum (Batra et al., 2003); Trichosanthes cucumerina var. anguina (Azeez & Morakinyo, 2004); Triticum aestivum (Stoddard and Marshall, 2005); Commiphora wightii (Soni, 2005); Glycine max (Xu et al., 2006); Oryza sativam (Nozu et al., 2006) and Arabidopsis sps. (Schlesier & Mock, 2006).
Variation in protein is also considered as an important biochemical event during growth and differentiation of cells (Hasegawa et al., 1979). There are several other evidences showing that protein synthesis occurs during tissue culture (Sardana, 1998; Audichya, 1999; Singh, 2004; Sharma, 2006).
In the present study, an attempt was made for quantitative estimation of protein and to determine the protein pattern profile in vivo (leaf, stem, fruit and root) and in vitro (differentiating and non-differentiating callus) tissues of Citrullus colocynthis by the use of spectrophotometric and electrophoretic techniques respectively.
SDS is an anionic detergent, which binds strongly to and denatures proteins. The number of SDS molecules bound to a polypeptide chain is approximately half the number of amino acid residues in that chain. The protein SDS-complex carries net negative charge, hence moves towards the anode and the separation is based on the size of protein. Proteins were separated by sodium dodecyl sulphatepolyacrylamide gel electrophoresis (Laemmli, 1970).
Reagents for SDS-PAGE
Preparation of reagents used in separating and stacking gel solutions
Solution A: 30 gm acrylamide + 0.8 gm N´,N´ methylene bisacrylamide dissolved in 100 ml of distilled water (stored at 4°C).
Solution B: 1.5 M Tris-HCl buffer (pH 8.8) 18.141 gm Tris (Tris hydroxy methyl aminomethane) dissolved in 50 ml of distilled water adjusted to pH 8.8 by concentrated HCl, volume made finally to 100 ml by adding distilled water.
Solution C: 10% SDS 10 gm SDS dissolved in 100 ml distilled water.
Solution D: 10% APS (Ammonium persulphate) 10 gm APS dissolved in 100 ml of distilled water (prepared fresh).
Solution E: Stock TEMED (N´,N´,N´,N´,-Tetramethyl ethylene diamine)
Solution F: 30 gm acrylamide + 1.6 gm N´, N´-methylene bisacrylamide Dissolved in 100 ml of distilled water
Solution G: 0.5 M Tris-HCl buffer 6.027 gm Tris-HCl in 50 ml of distilled water and pH (6.8) adjusted by distilled water concentrated HCl and volume made by adding distilled water up to 100 ml
Solution H: Electrode buffer pH (8.3) 3.025 gm Tris (Tris hydroxy methyl amino methane), 14.4 gm glycine and 1.0 gm SDS (Sodium dodecyl sulphate) Dissolved in one litre of distilled water (pH 8.3)
(This buffer was diluted when used).
Preparation of gel
Running gel was prepared by mixing the solutions in following ratio Table 1.
| Solutions | Volume (ml) |
|---|---|
| Distilled water | 12.1 ml |
| Solution-A | 10.0 ml |
| Solution-B | 7.5 ml |
| Solution-C | 0.3 ml |
| Solution-D | 0.1 ml |
| Solution-E | 0.01 ml |
The mixture was de-aerated before adding solution E (TEMED). The gel was caste in a manner as described above.
Stacking gel was prepared by mixing the solutions in following ratio Table 2.
| Solutions | Volume (ml) |
|---|---|
| Distilled water | 5.8 ml |
| Solution-C | 0.1 ml |
| Solution-D | 0.1 ml |
| Solution-E | 0.005 ml |
| Solution-F | 1.5 ml |
| Solution-G | 2.5 ml |
The mixture was poured over the running gel.
Preparation of protein sample for SDS-PAGE
Plant material (1 gm)
homogenized in 1 ml of 0.1 M phosphate buffer (pH 7.0 )
Centrifuged at 10,000 rpm for 20 minutes
Supernatant was collected
2.0 ml of supernatant was mixed with 2.0 ml of sample buffer
The mixture was boiled at 100°C for 3 minutes
Then cooled and centrifuged at 10,000 rpm for 10 minutes
Supernatant loaded on to gel
Preparation of sample buffer
2.0 ml of Glycerol + 2.0 ml of 0.5 N Tris (6.8 pH) + 0.1 ml of 20% SDS + 0.2 ml mercaptoethanol + 0.4 ml of 0.05% bromophenol blue (0.05 gm dissolve in 100 ml d.w.) + 2.4 ml of distilled water.
Protein visulalization (Staining and destaining)
The composition of the staining and destaining solution were adopted from Weber and Osborn (1969), except the methanol was omitted from destaining solution.
Preparation of Staining solution
0.25 gm Comassie Brilliant Blue R-50 (Sigma)
Dissolved in 43.0 ml of distilled water
50 ml methanol added + 7 ml of glacial acetic acid
Total volume was made up to 100 ml
Preparation of destaining solution: (7%) glacial acetic acid)
7 ml of glacial acetic acid + 93 ml distilled water
Procedure
(A) Pouring of the separating gel
1. The glass plate sandwich was fixed using two clean 0.75 mm spacers.
2. The sandwich was locked to the casting stand and sealed edges with agar.
3. Using the pasteur pipette, the separating gel solution was applied to the sandwich along an edge of one of the spacers.
4. Gel was polymerized in 30-60 minutes at room temperature
(B) Pouring of the stacking gel
1. After polymerization of separating gel pre-prepared stacking gel solution was poured into the centre of the sandwich.
2. Teflon comb (0.75 mm) was immediately inserted into the layer of stacking gel solution.
3. Stacking gel solution was polymerized for 1-2 hours at room temperature.
(C) Loading of the sample
1. After polymerization of stacking gel solution, comb was removed carefully.
2. The gel plate was assembled in the electrophoresis apparatus.
3. Electrode buffer was diluted before adding to the top and bottom reservoirs.
4. The samples (50-100 🕴l) were loaded with microsyringe.
(D) Running the gel
1. The gel was initially run at 50 V for 15-30 minutes and later continued at 100 V.
2. When bromophenol tracking dye had reached the bottom of the separating gel, the power supply was disconnected.
(E) Staining of the gel
1. The gel was carefully removed and incubated in 10% trichloro acetic acid (TCA) for 15 minutes.
2. After that, it was kept in staining solution for 16 hours at room temperature in a big petridish or tray.
(F) Destaining of the gel
1. The gel was destained by soaking it in destaining solution for 10-16 hours changing the destaining solution three or four times.
2. The destained gels were finally photographed. Diagramatic sketch of protein banding pattern (zymograms) were also made.
The protein bands were recorded on transparent sheets and were also photographed on trans-illuminator. Rm values of each band were calculated as the migration of protein band relative to the bromophenol marker dye movement. Diagrammatic sketch of protein banding pattern (zymograms) were also made by calculating the Rm value.
Rm = Distance moved by the protein
Distance travelled by the tracking dye
Results are presented in Table 3 and Plate-1, Figure 1.
| Protein bands | Leaf | Stem | Fruit | Root | Callus | Differentiating callus |
|---|---|---|---|---|---|---|
| 1 | 0.31 | 0.32 | 0.33 | 0.38 | 0.36 | 0.37 |
| 2 | - | - | - | - | 0.41 | 0.42 |
| 3 | 0.53 | 0.53 | 0.55 | 0.55 | 0.51 | 0.52 |
| 4 | 0.88 | 0.88 | 0.89 | 0.91 | 0.83 | 0.83 |
| 5 | - | - | - | - | 0.93 | 0.95 |
Differential banding pattern was observed in in vivo (leaf, stem, fruit and root) and in vitro (callus and differentiating callus) samples. In vivo samples (leaf, stem, fruit, and root) showed three bands while in vitro samples showed five bands. Variation in Rm value of these bands was marginal in all samples. Rm value of first upper band showed marginal variation in all samples (Rm value of leaf=0.31, stem=0.32, fruit=0.33, root=0.38, callus=0.36 and differentiating callus=0.37). In callus and differentiating callus, two bands was unique (Rm value of callus=0.41, 0.93 and Rm value of differentiating callus=0.42, 0.95).
Protein band with Rm value 0.53 was common in leaf and stem; and Rm value 0.55 was common in fruit and root but this band was different in callus (Rm value=0.51) and differentiating callus (Rm value=0.52). In callus and differentiating callus, protein band with Rm value 0.83 was common and Rm value 0.88 was common in leaf and stem but this band was different in root (Rm value=0.91) and fruit (Rm value=0.89).
Variation in intensity of protein bands was also observed. Differentiating callus showed three intense and two moderate bands while callus showed three intense bands and two faint bands. Fruit showed three intense bands while stem showed one intense, two moderate bands. Root showed two faint bands and one moderate band as compared to leaf (three faint bands).
Proteins are most abundant intracellular macromolecules and constitute over half the dry weight of most organisms. Proteins occupy a central position in the architecture and functioning of living matter. They are intimately connected with all phases of chemical and physical activity, that constitute the life of the cell. Protein contents have been estimated in various medicinal plant species by various workers such as Morus alba and Psoralea corylifolia (Singh, 2004); Bacopa monnieri (Mohapatra & Rath, 2005); Boerhaavia diffusa (Sharma, 2006); Balanities aegyptiaca (Bidawat, 2006); Berberis lyceum, Foeniculum vulgare and Justicia adhatoda (Uma et al., 2006).
Variation in protein content is also considered as an important biochemical event during growth and differentiation of cells (Audichya, 1999). In the present studies, among all the samples (in vivo and in vitro) tested, leaf showed higher protein level followed by fruit, root, stem, callus and differentiating callus. Similar to this Vijayvergia & Kumar (2007) reported maximum protein in leaf of Nerium indicum as compared to other intact plant parts. Similar findings were also reported by Arya (2007) in Pluchea lanceolata.
During the studies, it was found that protein content reduced during differentiation of shoots from callus. Similar results were obtained by Singh (2004) during callus differentiation of Morus alba and P. corylifolia. This reduction may be either due to effects of activity of other enzymes and the low level of storage protein contents (Jasrai et al., 1987) or the exogenous level of hormones supplied (Hasegawa et al., 1979). Contradictory to this, Kumar et al. (2007) reported higher level of proteins in differentiating callus as compared to callus.
Gel electrophoresis is a useful tool for characterizations of plant proteins. The recognitions of protein using electrophoresis is necessary and valuable for taxonomic studies in plants, animals, microorganisms and the viroids (Van den Berg, 2002). This technique has been widely used to study the protein diversity in many plant species i.e. Triticum aestivum (Stoddard and Marshall, 2005); Commiphora wightii (Soni, 2005); Glycine max (Xu et al., 2006); Oryza sativa (Nozu et al., 2006) and Arabidopsis (Schlesier & Mock, 2006). In the present study, differential banding pattern of protein in all in vivo (leaf, stem, fruit and root) and in vitro (callus and differentiating callus) samples was observed. In vivo samples (leaf, stem, fruit, and root) showed three bands while in vitro (callus and differentiating callus) samples showed five bands. Rm value of first upper band showed marginal variation in all samples (leaf, stem, fruit, root, callus and differentiating callus). Similarly Singh (2004) found higher number of protein bands in in vitro tissue of Morus alba and Psoralea corylifolia as compared to their in vitro tissue. Presence of more protein bands in callus and differentiating callus can be considered as a biochemical marker for callus and shoot bud initiation in this plant.
Differential banding pattern for protein in Citrullus Colocynthis (Linn.) Schrad. in all in vivo (leaf, stem, fruit and root) and in vitro (callus and differentiating callus) samples was observed. In vivo samples (leaf, stem, fruit and root) showed three bands while in vitro (callus and differentiating callus) samples showed five bands. Variation in intensity of protein bands was also observed in all samples. Fruit and in vitro samples showed more intense banding pattern. Rm value of first upper band showed marginal variation in all samples (leaf, stem, fruit, root, callus and differentiating callus).